Abstract
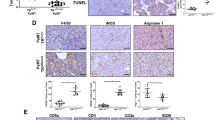
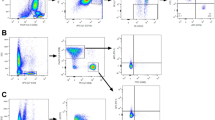
Surgery is essential for controlling the symptoms and complications of stage IV breast cancer. However, locoregional treatment of primary tumors often results in distant progression, including lung metastasis, the most common type of visceral metastasis. As a minimally invasive thermal therapy, microwave ablation (MWA) has been attempted in the treatment of breast cancer, but the innate immune response after MWA has not yet been reported. Using two murine models of stage IV breast cancer, we found that MWA of primary breast cancer inhibited the progression of lung metastasis and improved survival. NK cells were activated after MWA of the primary tumor and exhibited enhanced cytotoxic functions, and the cytotoxic pathways of NK cells were activated. Depletion experiments showed that NK cells but not CD4+ or CD8+ T cells played a pivotal role in prolonging survival. Then, we found that compared with surgery or control treatment, MWA of the primary tumor induced completely different NK-cell-related cytokine profiles. Macrophages were activated after MWA of the primary tumor and produced IL-15 that activated NK cells to inhibit the progression of metastasis. In addition, MWA of human breast cancer stimulated an autologous NK-cell response. These results demonstrate that MWA of the primary tumor in metastatic breast cancer inhibits metastatic progression via the macrophage/IL-15/NK-cell axis. MWA of the primary tumor may be a promising treatment strategy for de novo stage IV breast cancer, although further substantiation is essential for clinical testing.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Harris, E., Barry, M. & Kell, M. R. Meta-analysis to determine if surgical resection of the primary tumour in the setting of stage IV breast cancer impacts on survival. Ann. Surg. Oncol. 20, 2828–2834 (2013).
Bafford, A. C. et al. Breast surgery in stage IV breast cancer: impact of staging and patient selection on overall survival. Breast Cancer Res Treat. 115, 7–12 (2009).
Soran A. et al. Randomized trial comparing resection of primary tumor with no surgery in stage IV breast cancer at presentation: protocol MF07-01. Ann Surg Oncol. 25, 3141–3149 (2018).
Badwe, R. et al. Locoregional treatment versus no treatment of the primary tumour in metastatic breast cancer: an open-label randomised controlled trial. Lancet Oncol. 16, 1380–1388 (2015).
Gunduz, N., Fisher, B. & Saffer, E. A. Effect of surgical removal on the growth and kinetics of residual tumor. Cancer Res. 39, 3861–3865 (1979).
Fisher, B., Gunduz, N., Coyle, J., Rudock, C. & Saffer, E. Presence of a growth-stimulating factor in serum following primary tumor removal in mice. Cancer Res. 49, 1996–2001 (1989).
Demicheli, R., Retsky, M. W., Swartzendruber, D. E. & Bonadonna, G. Proposal for a new model of breast cancer metastatic development. Ann. Oncol. 8, 1075–1080 (1997).
Al-Sahaf, O., Wang, J. H., Browne, T. J., Cotter, T. G. & Redmond, H. P. Surgical injury enhances the expression of genes that mediate breast cancer metastasis to the lung. Ann. Surg. 252, 1037–1043 (2010).
Krall J. A. et al. The systemic response to surgery triggers the outgrowth of distant immune-controlled tumors in mouse models of dormancy. Sci Transl Med. 10, eaan3464 (2018).
Coffey, J. C. et al. Excisional surgery for cancer cure: therapy at a cost. Lancet Oncol. 4, 760–768 (2003).
O’Reilly, M. S. et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 88, 277–285 (1997).
Maniwa, Y., Kanki, M. & Okita, Y. Importance of the control of lung recurrence soon after surgery of pulmonary metastases. Am. J. Surg. 179, 122–125 (2000).
Lange, P. H., Hekmat, K., Bosl, G., Kennedy, B. J. & Fraley, E. E. Acclerated growth of testicular cancer after cytoreductive surgery. Cancer 45, 1498–1506 (1980).
Dromi, S. A. et al. Radiofrequency ablation induces antigen-presenting cell infiltration and amplification of weak tumor-induced immunity. Radiology 251, 58–66 (2009).
Zerbini, A. et al. Radiofrequency thermal ablation for hepatocellular carcinoma stimulates autologous NK-cell response. Gastroenterology 138, 1931–1942 (2010).
Zerbini, A. et al. Radiofrequency thermal ablation of hepatocellular carcinoma liver nodules can activate and enhance tumor-specific T-cell responses. Cancer Res. 66, 1139–1146 (2006).
Behm, B. et al. Additive antitumour response to the rabbit VX2 hepatoma by combined radio frequency ablation and toll like receptor 9 stimulation. Gut 65, 134–143 (2016).
Chu, K. F. & Dupuy, D. E. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat. Rev. Cancer 14, 199–208 (2014).
Xu, A. et al. TLR9 agonist enhances radiofrequency ablation-induced CTL responses, leading to the potent inhibition of primary tumor growth and lung metastasis. Cell Mol. Immunol. 16, 820–832 (2019).
Sanchez-Ortiz, R. F., Tannir, N., Ahrar, K. & Wood, C. G. Spontaneous regression of pulmonary metastases from renal cell carcinoma after radio frequency ablation of primary tumor: an in situ tumor vaccine? J. Urol. 170, 178–179 (2003).
Kim, H., Park, B. K. & Kim, C. K. Spontaneous regression of pulmonary and adrenal metastases following percutaneous radiofrequency ablation of a recurrent renal cell carcinoma. Korean J. Radiol. 9, 470–472 (2008).
Soanes, W. A., Ablin, R. J. & Gonder, M. J. Remission of metastatic lesions following cryosurgery in prostatic cancer: immunologic considerations. J. Urol. 104, 154–159 (1970).
Zhou, W. et al. US-guided percutaneous microwave coagulation of small breast cancers: a clinical study. Radiology 263, 364–373 (2012).
Burak, W. E. Jr et al. Radiofrequency ablation of invasive breast carcinoma followed by delayed surgical excision. Cancer 98, 1369–1376 (2003).
Manenti, G. et al. Small breast cancers: in vivo percutaneous US-guided radiofrequency ablation with dedicated cool-tip radiofrequency system. Radiology 251, 339–346 (2009).
Palussiere, J. et al. Radiofrequency ablation as a substitute for surgery in elderly patients with nonresected breast cancer: pilot study with long-term outcomes. Radiology 264, 597–605 (2012).
Roubidoux, M. A. et al. Small (< 2.0-cm) breast cancers: mammographic and US findings at US-guided cryoablation–initial experience. Radiology 233, 857–867 (2004).
Simon, C. J., Dupuy, D. E. & Mayo-Smith, W. W. Microwave ablation: principles and applications. Radiographics 25(Suppl 1), S69–S83 (2005).
Zhou, W. et al. Comparison of ablation zones among different tissues using 2450-MHz cooled-shaft microwave antenna: results in ex vivo porcine models. PLoS ONE 8, e71873 (2013).
Zhou, W. et al. Image and pathological changes after microwave ablation of breast cancer: a pilot study. Eur. J. Radiol. 83, 1771–1777 (2014).
Li, L. et al. Microwave ablation combined with OK-432 induces Th1-type response and specific antitumor immunity in a murine model of breast cancer. J. Transl. Med. 15, 23 (2017).
Zhu, J. et al. Enhanced antitumor efficacy through microwave ablation in combination with immune checkpoints blockade in breast cancer: a pre-clinical study in a murine model. Diagn. Inter. Imaging 99, 135–142 (2018).
Todorova, V. K., Klimberg, V. S., Hennings, L., Kieber-Emmons, T. & Pashov, A. Immunomodulatory effects of radiofrequency ablation in a breast cancer model. Immunol. Investig. 39, 74–92 (2010).
Danna, E. A. et al. Surgical removal of primary tumor reverses tumor-induced immunosuppression despite the presence of metastatic disease. Cancer Res. 64, 2205–2211 (2004).
Pulaski, B. A. & Ostrand-Rosenberg, S. Reduction of established spontaneous mammary carcinoma metastases following immunotherapy with major histocompatibility complex class II and B7.1 cell-based tumor vaccines. Cancer Res. 58, 1486–1493 (1998).
Habif, G., Crinier, A., Andre, P., Vivier, E. & Narni-Mancinelli, E. Targeting natural killer cells in solid tumors. Cell Mol. Immunol. 16, 415–422 (2019).
Sivori, S. et al. Human NK cells: surface receptors, inhibitory checkpoints, and translational applications. Cell Mol. Immunol. 16, 430–441 (2019).
Banh, C., Miah, S. M., Kerr, W. G. & Brossay, L. Mouse natural killer cell development and maturation are differentially regulated by SHIP-1. Blood 120, 4583–4590 (2012).
Young, H. A. & Ortaldo, J. Cytokines as critical co-stimulatory molecules in modulating the immune response of natural killer cells. Cell Res. 16, 20–24 (2006).
Steel, J. C., Waldmann, T. A. & Morris, J. C. Interleukin-15 biology and its therapeutic implications in cancer. Trends Pharm. Sci. 33, 35–41 (2012).
Gerratana, L. et al. Pattern of metastasis and outcome in patients with breast cancer. Clin. Exp. Metastasis 32, 125–133 (2015).
Gu, Y., Wu, G., Zou, X., Huang, P. & Yi, L. Prognostic value of site-specific metastases and surgery in de novo stage IV triple-negative breast cancer: a population-based analysis. Med. Sci. Monit. 26, e920432 (2020).
Kassam, F. et al. Survival outcomes for patients with metastatic triple-negative breast cancer: implications for clinical practice and trial design. Clin. Breast Cancer 9, 29–33 (2009).
Pulaski B. A., Ostrand-Rosenberg S. Mouse 4T1 breast tumor model. Curr. Protoc. Immunol. Chapter 20, Unit 20.2 (2001).
Ahmad, F. et al. Changes in interleukin-1beta and 6 after hepatic microwave tissue ablation compared with radiofrequency, cryotherapy and surgical resections. Am. J. Surg. 200, 500–506 (2010).
Dong, B. W. et al. Sequential pathological and immunologic analysis of percutaneous microwave coagulation therapy of hepatocellular carcinoma. Int. J. Hyperth. 19, 119–133 (2003).
Jansen, M. C. et al. Cryoablation induces greater inflammatory and coagulative responses than radiofrequency ablation or laser induced thermotherapy in a rat liver model. Surgery 147, 686–695 (2010).
Chapman, W. C. et al. Hepatic cryoablation, but not radiofrequency ablation, results in lung inflammation. Ann. Surg. 231, 752–761 (2000).
Rashid, O. M. et al. Resection of the primary tumor improves survival in metastatic breast cancer by reducing overall tumor burden. Surgery 153, 771–778 (2013).
Vivier, E., Tomasello, E., Baratin, M., Walzer, T. & Ugolini, S. Functions of natural killer cells. Nat. Immunol. 9, 503–510 (2008).
Gallucci, S., Lolkema, M. & Matzinger, P. Natural adjuvants: endogenous activators of dendritic cells. Nat. Med. 5, 1249–1255 (1999).
Sauter, B. et al. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J. Exp. Med. 191, 423–434 (2000).
Walzer, T., Dalod, M., Robbins, S. H., Zitvogel, L. & Vivier, E. Natural-killer cells and dendritic cells: “l’union fait la force”. Blood 106, 2252–2258 (2005).
Chavez, M. et al. Distinct immune signatures in directly treated and distant tumors result from TLR adjuvants and focal ablation. Theranostics 8, 3611–3628 (2018).
Kiniwa, T. et al. NK cells activated by Interleukin-4 in cooperation with Interleukin-15 exhibit distinctive characteristics. Proc. Natl Acad. Sci. USA 113, 10139–10144 (2016).
Zhang, M. et al. IL-15 enhanced antibody-dependent cellular cytotoxicity mediated by NK cells and macrophages. Proc. Natl Acad. Sci. USA 115, E10915–E10924 (2018).
Yoshimoto, T. et al. IL-12 up-regulates IL-18 receptor expression on T cells, Th1 cells, and B cells: synergism with IL-18 for IFN-gamma production. J. Immunol. 161, 3400–3407 (1998).
Malek, T. R., Yu, A., Scibelli, P., Lichtenheld, M. G. & Codias, E. K. Broad programming by IL-2 receptor signaling for extended growth to multiple cytokines and functional maturation of antigen-activated T cells. J. Immunol. 166, 1675–1683 (2001).
Acknowledgements
This work was supported in part by the National Natural Science Foundation of China (81771953), the Six Kinds of Outstanding Talent Foundation of Jiangsu Province (WSW-014, to W.Z.), the Natural Science Foundation of Jiangsu Province (BK20180108), and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Contributions
W.Z. and S.W. contributed to the conception and design of the study, the analysis and interpretation of data and the revision of the article, and provided final approval of the version to be submitted. M.Y., H.P., L.L., C.W., Y.W., G.M., M.Q., and J.L. performed the experimental study and statistical analysis and drafted and revised the article. N.C., M.Z., H.X., L.L., X.G., and Y.Z. participated in the clinical study, performed the statistical analysis, and drafted and revised the article. All authors read and approved the final version of the manuscript. M.Y., H.P., and L.L. contributed equally to this work. We would like to thank all the members of the SW and NC laboratories for helpful discussions and comments.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Yu, M., Pan, H., Che, N. et al. Microwave ablation of primary breast cancer inhibits metastatic progression in model mice via activation of natural killer cells. Cell Mol Immunol 18, 2153–2164 (2021). https://doi.org/10.1038/s41423-020-0449-0
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41423-020-0449-0
Keywords
This article is cited by
-
Heterogeneous tissue-specific macrophages orchestrate metastatic organotropism of breast cancer: implications for promising therapeutics
Journal of Translational Medicine (2025)
-
Microwave ablation of non-small cell lung cancer enhances local T-cell abundance and alters monocyte interactions
BMC Cancer (2025)
-
Survival outcomes and optimal candidates for primary tumor resection in head and neck squamous cell carcinoma with distant metastasis at initial diagnosis
European Archives of Oto-Rhino-Laryngology (2025)
-
An insight into the role of innate immune cells in breast tumor microenvironment
Breast Cancer (2025)
-
Combination of percutaneous thermal ablation and adoptive Th9 cell transfer therapy against non-small cell lung cancer
Experimental Hematology & Oncology (2024)