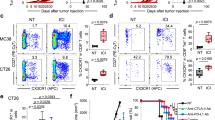
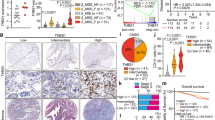
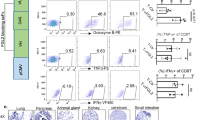
Abstract
Brain tumors such as glioblastomas are resistant to immune checkpoint blockade therapy, largely due to limited T cell infiltration in the tumors. Here, we show that mice bearing intracranial tumors exhibit systemic immunosuppression and T cell sequestration in bone marrow, leading to reduced T cell infiltration in brain tumors. Elevated plasma corticosterone drives the T cell sequestration via glucocorticoid receptors in tumor-bearing mice. Immunosuppression mediated by glucocorticoid-induced T cell dynamics and the subsequent tumor growth promotion can be abrogated by adrenalectomy, the administration of glucocorticoid activation inhibitors or glucocorticoid receptor antagonists, and in mice with T cell-specific deletion of glucocorticoid receptor. CCR8 expression in T cells is increased in tumor-bearing mice in a glucocorticoid receptor-dependent manner. Additionally, chemokines CCL1 and CCL8, the ligands for CCR8, are highly expressed in bone marrow immune cells in tumor-bearing mice to recruit T cells. These findings suggested that brain tumor-induced glucocorticoid surge and CCR8 upregulation in T cells lead to T cell sequestration in bone marrow, impairing the anti-tumor immune response. Targeting the glucocorticoid receptor-CCR8 axis may offer a promising immunotherapeutic approach for the treatment of intracranial tumors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Ostrom QT, Gittleman H, Liao P, Vecchione-Koval T, Wolinsky Y, Kruchko C, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro Oncol. 2017;19:v1–v88.
Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the metropolitan detroit cancer surveillance system. J Clin Oncol. 2004;22:2865–72.
Sampson JH, Gunn MD, Fecci PE, Ashley DM. Brain immunology and immunotherapy in brain tumours. Nat Rev Cancer. 2020;20:12–25.
Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23:1231–51.
Buerki RA, Chheda ZS, Okada H. Immunotherapy of primary brain tumors: facts and hopes. Clin Cancer Res. 2018;24:5198–205.
Chongsathidkiet P, Jackson C, Koyama S, Loebel F, Cui X, Farber SH, et al. Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nat Med. 2018;24:1459–68.
DeCordova S, Shastri A, Tsolaki AG, Yasmin H, Klein L, Singh SK, et al. Molecular heterogeneity and immunosuppressive microenvironment in glioblastoma. Front Immunol. 2020;11:1402.
Liebner S, Dijkhuizen RM, Reiss Y, Plate KH, Agalliu D, Constantin G. Functional morphology of the blood-brain barrier in health and disease. Acta Neuropathol. 2018;135:311–36.
Ryken TC, McDermott M, Robinson PD, Ammirati M, Andrews DW, Asher AL, et al. The role of steroids in the management of brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96:103–14.
Pitter KL, Tamagno I, Alikhanyan K, Hosni-Ahmed A, Pattwell SS, Donnola S, et al. Corticosteroids compromise survival in glioblastoma. Brain. 2016;139:1458–71.
Yang H, Xia L, Chen J, Zhang S, Martin V, Li Q, et al. Stress-glucocorticoid-TSC22D3 axis compromises therapy-induced antitumor immunity. Nat Med. 2019;25:1428–41.
Acharya N, Madi A, Zhang H, Klapholz M, Escobar G, Dulberg S, et al. Endogenous glucocorticoid signaling regulates CD8(+) T cell differentiation and development of dysfunction in the tumor microenvironment. Immunity. 2020;53:658–71.e656
Collins N, Han SJ, Enamorado M, Link VM, Huang B, Moseman EA, et al. The bone marrow protects and optimizes immunological memory during dietary restriction. Cell. 2019;178:1088–101.e1015
Cain DW, Cidlowski JA. Immune regulation by glucocorticoids. Nat Rev Immunol. 2017;17:233–47.
Sidler D, Renzulli P, Schnoz C, Berger B, Schneider-Jakob S, Flück C, et al. Colon cancer cells produce immunoregulatory glucocorticoids. Oncogene. 2011;30:2411–9.
Islam SA, Ling MF, Leung J, Shreffler WG, Luster AD. Identification of human CCR8 as a CCL18 receptor. J Exp Med. 2013;210:1889–98.
Farmaki E, Kaza V, Papavassiliou AG, Chatzistamou I, Kiaris H. Induction of the MCP chemokine cluster cascade in the periphery by cancer cell-derived Ccl3. Cancer Lett. 2017;389:49–58.
Hou PP, Luo LJ, Chen HZ, Chen QT, Bian XL, Wu SF, et al. Ectosomal PKM2 promotes HCC by inducing macrophage differentiation and remodeling the tumor microenvironment. Mol Cell. 2020;78:1192–206.e1110
Knipfer L, Schulz-Kuhnt A, Kindermann M, Greif V, Symowski C, Voehringer D, et al. A CCL1/CCR8-dependent feed-forward mechanism drives ILC2 functions in type 2-mediated inflammation. J Exp Med. 2019;216:2763–77.
Chen XJ, Wei WF, Wang ZC, Wang N, Guo CH, Zhou CF, et al. A novel lymphatic pattern promotes metastasis of cervical cancer in a hypoxic tumour-associated macrophage-dependent manner. Angiogenesis. 2021;24:549–65.
Lim JY, Ryu DB, Kim TW, Lee SE, Park G, Yoon HK, et al. CCL1 blockade alleviates human mesenchymal stem cell (hMSC)-induced pulmonary fibrosis in a murine sclerodermatous graft-versus-host disease (Scl-GVHD) model. Stem Cell Res Ther. 2020;11:254.
King LB, Vacchio MS, Dixon K, Hunziker R, Margulies DH, Ashwell JD. A targeted glucocorticoid receptor antisense transgene increases thymocyte apoptosis and alters thymocyte development. Immunity. 1995;3:647–56.
Lu FW, Yasutomo K, Goodman GB, McHeyzer-Williams LJ, McHeyzer-Williams MG, Germain RN, et al. Thymocyte resistance to glucocorticoids leads to antigen-specific unresponsiveness due to “holes” in the T cell repertoire. Immunity. 2000;12:183–92.
Rocamora-Reverte L, Reichardt HM, Villunger A, Wiegers G. T-cell autonomous death induced by regeneration of inert glucocorticoid metabolites. Cell Death Dis. 2017;8:e2948.
Li L, Zhang T, Diao W, Jin F, Shi L, Meng J, et al. Role of myeloid-derived suppressor cells in glucocorticoid-mediated amelioration of FSGS. J Am Soc Nephrol. 2015;26:2183–97.
Schweingruber N, Fischer HJ, Fischer L, van den Brandt J, Karabinskaya A, Labi V, et al. Chemokine-mediated redirection of T cells constitutes a critical mechanism of glucocorticoid therapy in autoimmune CNS responses. Acta Neuropathol. 2014;127:713–29.
Mittelstadt PR, Monteiro JP, Ashwell JD. Thymocyte responsiveness to endogenous glucocorticoids is required for immunological fitness. J Clin Invest. 2012;122:2384–94.
Shimba A, Cui G, Tani-Ichi S, Ogawa M, Abe S, Okazaki F, et al. Glucocorticoids drive diurnal oscillations in T cell distribution and responses by inducing interleukin-7 receptor and CXCR4. Immunity. 2018;48:286–98.e286
Volden PA, Conzen SD. The influence of glucocorticoid signaling on tumor progression. Brain Behav Immun. 2013;30 Suppl:S26–31.
Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer Inst. 2000;92:994–1000.
Kertser A, Baruch K, Deczkowska A, Weiner A, Croese T, Kenigsbuch M, et al. Corticosteroid signaling at the brain-immune interface impedes coping with severe psychological stress. Sci Adv. 2019;5:eaav4111.
Winkler F, Venkatesh HS, Amit M, Batchelor T, Demir IE, Deneen B, et al. Cancer neuroscience: state of the field, emerging directions. Cell. 2023;186:1689–707.
Seano G, Nia HT, Emblem KE, Datta M, Ren J, Krishnan S, et al. Solid stress in brain tumours causes neuronal loss and neurological dysfunction and can be reversed by lithium. Nat Biomed Eng. 2019;3:230–45.
Momin A, Bahrampour S, Min HK, Chen X, Wang X, Sun Y, et al. Channeling force in the brain: mechanosensitive ion channels choreograph mechanics and malignancies. Trends Pharm Sci. 2021;42:367–84.
Ayasoufi K, Pfaller CK, Evgin L, Khadka RH, Tritz ZP, Goddery EN, et al. Brain cancer induces systemic immunosuppression through release of non-steroid soluble mediators. Brain. 2020;143:3629–52.
Xiong SY, Wen HZ, Dai LM, Lou YX, Wang ZQ, Yi YL, et al. A brain-tumor neural circuit controls breast cancer progression in mice. J Clin Invest. 2023;133:e167725b.
Song L, Cao L, Liu R, Ma H, Li Y, Shang Q, et al. The critical role of T cells in glucocorticoid-induced osteoporosis. Cell Death Dis. 2020;12:45.
Guo B, Huang X, Cooper S, Broxmeyer HE. Glucocorticoid hormone-induced chromatin remodeling enhances human hematopoietic stem cell homing and engraftment. Nat Med. 2017;23:424–8.
Zhang L, Prak L, Rayon-Estrada V, Thiru P, Flygare J, Lim B, et al. ZFP36L2 is required for self-renewal of early burst-forming unit erythroid progenitors. Nature. 2013;499:92–96.
Flygare J, Rayon Estrada V, Shin C, Gupta S, Lodish HF. HIF1alpha synergizes with glucocorticoids to promote BFU-E progenitor self-renewal. Blood. 2011;117:3435–44.
Ozga AJ, Chow MT, Luster AD. Chemokines and the immune response to cancer. Immunity. 2021;54:859–74.
Eruslanov E, Stoffs T, Kim WJ, Daurkin I, Gilbert SM, Su LM, et al. Expansion of CCR8(+) inflammatory myeloid cells in cancer patients with urothelial and renal carcinomas. Clin Cancer Res. 2013;19:1670–80.
Das S, Sarrou E, Podgrabinska S, Cassella M, Mungamuri SK, Feirt N, et al. Tumor cell entry into the lymph node is controlled by CCL1 chemokine expressed by lymph node lymphatic sinuses. J Exp Med. 2013;210:1509–28.
Ruckes T, Saul D, Van Snick J, Hermine O, Grassmann R. Autocrine antiapoptotic stimulation of cultured adult T-cell leukemia cells by overexpression of the chemokine I-309. Blood. 2001;98:1150–9.
Gajewski TF, Corrales L, Williams J, Horton B, Sivan A, Spranger S. Cancer immunotherapy targets based on understanding the T cell-inflamed versus non-T cell-inflamed tumor microenvironment. Adv Exp Med Biol. 2017;1036:19–31.
Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14:1014–22.
Keskin DB, Anandappa AJ, Sun J, Tirosh I, Mathewson ND, Li S, et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature. 2019;565:234–9.
Wang T, Zhang H, Qiu W, Han Y, Liu H, Li Z. Biomimetic nanoparticles directly remodel immunosuppressive microenvironment for boosting glioblastoma immunotherapy. Bioact Mater. 2022;16:418–32.
Berenguer J, Lagerweij T, Zhao XW, Dusoswa S, van der Stoop P, Westerman B, et al. Glycosylated extracellular vesicles released by glioblastoma cells are decorated by CCL18 allowing for cellular uptake via chemokine receptor CCR8. J Extracell Vesicles. 2018;7:1446660.
Choi S, Zhang B, Ma S, Gonzalez-Celeiro M, Stein D, Jin X, et al. Corticosterone inhibits GAS6 to govern hair follicle stem-cell quiescence. Nature. 2021;592:428–32.
Acknowledgements
This study was supported by grants from the National Key R&D Program of China (2022YFA0807300 and 2021YFA1100600), the National Natural Science Foundation of China (81930085 and 32150710523), the Jiangsu Province International Joint Laboratory for Regenerative Medicine Fund and Suzhou Science and Technology Bureau (ZXL2021440, SWY202202 and SYS2020087).
Author information
Authors and Affiliations
Contributions
JZ performed most of the experiments, analyzed the data, and wrote the manuscript. YS and XX produced the mouse models and assisted with the experiments. WB, YL and TY assisted with the experiments. LC, JF, PL, YC and ZL provided reagents and advice. CS and YS supervised the project, designed the experiments, and together edited the manuscript. All authors have read and approved the article. All of the schematic representation are prepared using the BioRender online website (https://www.biorender.com).
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests. YS is editorial board member of Cellular & Molecular Immunology, but he has not been involved in the peer review or the decision-making of the article.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, J., Shi, Y., Xue, X. et al. Targeting the glucocorticoid receptor-CCR8 axis mediated bone marrow T cell sequestration enhances infiltration of anti-tumor T cells in intracranial cancers. Cell Mol Immunol 21, 1145–1157 (2024). https://doi.org/10.1038/s41423-024-01202-5
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41423-024-01202-5