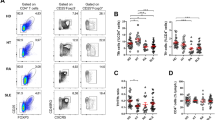
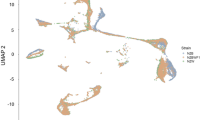
Abstract
T follicular helper (Tfh) cells specialize in facilitating germinal center B-cell activation and high-affinity antibody generation, which are crucial in humoral immune responses. However, aberrant control of Tfh cells also contributes to the generation of self-reactive autoantibodies and promotes autoimmune diseases such as systemic lupus erythematosus (SLE). The mechanisms that control proper Tfh expansion remain unclear. Here, we show that farnesoid X receptor (FXR) is relatively upregulated in Tfh cells. Genetic deletion of Fxr restrains Tfh expansion both at steady state and in pristane-induced lupus. As a consequence of these defects, mice lacking Fxr manifested GC dysfunction and decreased plasma cell and autoantibody production, which alleviated nephritis progression in pristane-induced lupus. Mechanistically, FXR intrinsically regulates cholesterol homeostasis in Tfh cells, which subsequently controls Tfh cell proliferation. Preclinical treatment of wild-type (WT) mice with the clinically approved drug ursodeoxycholic acid (UDCA) to reduce FXR signaling mitigated lupus disease progression by repressing Tfh expansion, the GC reaction and autoantibody production. These findings provide a rationale for exploring FXR as a potential therapeutic target for SLE.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Source sequencing data and supporting analytic code for this article will be made available upon reasonable request to the lead contact.
References
Yu D, Rao S, Tsai LM, Lee SK, He Y, Sutcliffe EL, et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009;31:457–68.
Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–10.
Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, et al. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325:1001–5.
Deenick EK, Chan A, Ma CS, Gatto D, Schwartzberg PL, Brink R, et al. Follicular helper T-cell differentiation requires continuous antigen presentation that is independent of unique B-cell signaling. Immunity. 2010;33:241–53.
Vinuesa CG, Cyster JG. How T cells earn the follicular rite of passage. Immunity. 2011;35:671–80.
Choi YS, Kageyama R, Eto D, Escobar TC, Johnston RJ, Monticelli L, et al. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity. 2011;34:932–46.
Harker JA, Lewis GM, Mack L, Zuniga EI. Late interleukin-6 escalates T follicular helper cell responses and controls a chronic viral infection. Science. 2011;334:825–9.
Ozaki K, Spolski R, Ettinger R, Kim H-P, Wang G, Qi C-F, et al. Regulation of B-cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J Immunol. 2004;173:5361–71.
Gensous N, Charrier M, Duluc D, Contin-Bordes C, Truchetet M-E, Lazaro E, et al. T follicular helper cells in autoimmune disorders. Front Immunol. 2018;9:1637.
He J, Tsai LM, Leong YA, Hu X, Ma CS, Chevalier N, et al. Circulating precursor CCR7(lo)PD-1(hi) CXCR5+ CD4+ T cells indicate Tfh cell activity and promote antibody responses upon antigen reexposure. Immunity. 2013;39:770–81.
Linterman MA, Rigby RJ, Wong RK, Yu D, Brink R, Cannons JL, et al. Follicular helper T cells are required for systemic autoimmunity. J Exp Med. 2009;206:561–76.
Mittereder N, Kuta E, Bhat G, Dacosta K, Cheng LI, Herbst R, et al. Loss of immune tolerance is controlled by ICOS in Sle1 mice. J Immunol. 2016;197:491–503.
Kim SJ, Schätzle S, Ahmed SS, Haap W, Jang SH, Gregersen PK, et al. Increased cathepsin S in Prdm1-/- dendritic cells alters the TFH cell repertoire and contributes to lupus. Nat Immunol. 2017;18:1016–24.
Gao X, Song Y, Du P, Yang S, Cui H, Lu S, et al. Administration of a microRNA-21 inhibitor improves the lupus-like phenotype in MRL/lpr mice by repressing Tfh cell-mediated autoimmune responses. Int Immunopharmacol. 2022;106:108578.
Gao X, Song Y, Wu J, Lu S, Min X, Liu L, et al. Iron-dependent epigenetic modulation promotes pathogenic T-cell differentiation in lupus. J Clin Invest. 2022;132:e152345.
Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, et al. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–5.
Edwards PA, Kast HR, Anisfeld AM. BAREing it all: the adoption of LXR and FXR and their roles in lipid homeostasis. J Lipid Res. 2002;43:2–12.
Yang F, Huang X, Yi T, Yen Y, Moore DD, Huang W. Spontaneous development of liver tumors in the absence of the bile acid receptor farnesoid X receptor. Cancer Res. 2007;67:863–7.
Inagaki T, Moschetta A, Lee Y-K, Peng L, Zhao G, Downes M, et al. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci USA. 2006;103:3920–5.
Gadaleta RM, van Erpecum KJ, Oldenburg B, Willemsen ECL, Renooij W, Murzilli S, et al. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut. 2011;60:463–72.
Fu T, Coulter S, Yoshihara E, Oh TG, Fang S, Cayabyab F, et al. FXR regulates intestinal cancer stem cell proliferation. Cell. 2019;176:1098–1112.e18.
Sarkissian T, Beyenne J, Feldman B, Adeli K, Silverman E. The complex nature of the interaction between disease activity and therapy on the lipid profile in patients with pediatric systemic lupus erythematosus. Arthritis Rheum. 2006;54:1283–90.
Hu J-Q, Yan Y-H, Xie H, Feng X-B, Ge W-H, Zhou H, et al. Targeting abnormal lipid metabolism of T cells for systemic lupus erythematosus treatment. Biomed Pharmacother. 2023;165:115198.
Lee SK, Rigby RJ, Zotos D, Tsai LM, Kawamoto S, Marshall JL, et al. B-cell priming for extrafollicular antibody responses requires Bcl-6 expression by T cells. J Exp Med. 2011;208:1377–88.
Luo J, Jiang L-Y, Yang H, Song B-L. Intracellular cholesterol transport by sterol transfer proteins at membrane contact sites. Trends Biochem Sci. 2019;44:273–92.
Luo J, Yang H, Song B-L. Mechanisms and regulation of cholesterol homeostasis. Nat Rev Mol Cell Biol. 2020;21:225–45.
Wang Y-D, Chen W-D, Moore DD, Huang W. FXR: a metabolic regulator and cell protector. Cell Res. 2008;18:1087–95.
Rawlings DJ, Metzler G, Wray-Dutra M, Jackson SW. Altered B-cell signaling in autoimmunity. Nat Rev Immunol. 2017;17:421–36.
Gatto D, Brink R. The germinal center reaction. J Allergy Clin Immunol. 2010;126:898–907.
Sage PT, Sharpe AH. T follicular regulatory cells in the regulation of B-cell responses. Trends Immunol. 2015;36:410–8.
Crotty S. T follicular helper cell biology: a decade of discovery and diseases. Immunity. 2019;50:1132–48.
Suwannaroj S, Lagoo A, McMurray RW. Suppression of renal disease and mortality in the female NZB x NZW F1 mouse model of systemic lupus erythematosus (SLE) by chenodeoxycholic acid. Lupus. 2001;10:562–7.
Dawson PA, Karpen SJ. Intestinal transport and metabolism of bile acids. J Lipid Res. 2015;56:1085–99.
Ahmad TR, Haeusler RA. Bile acids in glucose metabolism and insulin signaling - mechanisms and research needs. Nat Rev Endocrinol. 2019;15:701–12.
Clifford BL, Sedgeman LR, Williams KJ, Morand P, Cheng A, Jarrett KE, et al. FXR activation protects against NAFLD via bile-acid-dependent reductions in lipid absorption. Cell Metab. 2021;33:1671–84.e4.
Xu H, Fang F, Wu K, Song J, Li Y, Lu X, et al. Gut microbiota-bile acid crosstalk regulates murine lipid metabolism via the intestinal FXR-FGF19 axis in diet-induced humanized dyslipidemia. Microbiome. 2023;11:262.
Li T, Matozel M, Boehme S, Kong B, Nilsson L-M, Guo G, et al. Overexpression of cholesterol 7α-hydroxylase promotes hepatic bile acid synthesis and secretion and maintains cholesterol homeostasis. Hepatology. 2011;53:996–1006.
Fu G, Guy CS, Chapman NM, Palacios G, Wei J, Zhou P, et al. Metabolic control of TFH cells and humoral immunity by phosphatidylethanolamine. Nature. 2021;595:724–9.
Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725.
Cao Y, Yang Q, Deng H, Tang J, Hu J, Liu H, et al. Transcription factor ATF3 protects against colitis by regulating follicular helper T cells in Peyer’s patches. Proc Natl Acad Sci USA. 2019;116:6286–91.
Cao Y, Li Y, Wang X, Liu S, Zhang Y, Liu G, et al. Dopamine inhibits group 2 innate lymphoid cell-driven allergic lung inflammation by dampening mitochondrial activity. Immunity. 2023;56:320–35.e9.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (82271866, 82471853 and 82402127), the Guangzhou Key Research and Development Program (02A004999000186), and the Guangdong Basic and Applied Basic Research Foundation (2024B1515020039, 2021A1515011451, 2017B030314120 and 202201011028).
Author information
Authors and Affiliations
Contributions
XW and LY conceptualized and investigated the studies; XW, SL, and YZ analyzed the data and interpreted the results. XW, LY, SF, SL, YZ, LZ, WH, JS (Jiawei Song), JS (Jingxuan Shao) and FW performed the experiments. CZ, XL, SZ and YX collected and analyzed the clinical samples. XW conducted the analysis and interpretation of the RNA-seq data. XW, XC and YC wrote the manuscript. YC, JZ and LY conceived the studies, interpreted the data, directed the studies, and revised the manuscript. XW, LY, SL and YZ share co-first authorship, and the order in which they are listed is determined by their workload.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests. JZ is an editorial board member of Cellular & Molecular Immunology, but she has not been involved in peer review or decision-making related to the article.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
41423_2025_1309_MOESM3_ESM.jpg
Supplementary Figure3. CD4creFxrfl/fl mice exhibits increased serum autoantibodies and splenic plasma cells in the pristane-induced lupus model
41423_2025_1309_MOESM7_ESM.jpg
Supplementary Figure7. FXR signaling specifically modulated Tfh cell development by controlling the intracellular cholesterol metabolism
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, X., Ye, L., Liu, S. et al. FXR inhibition functions as a checkpoint blockade of the pathogenic Tfh cell response in lupus. Cell Mol Immunol 22, 889–900 (2025). https://doi.org/10.1038/s41423-025-01309-3
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41423-025-01309-3
Keywords
This article is cited by
-
Cholesterol promotes autoimmune pathology through T follicular helper cells
Cellular & Molecular Immunology (2025)