Abstract
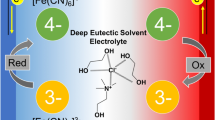
A p-type supramolecular thermocell was constructed with a ferrocenecarboxylate/ferroceniumcarboxylate pair. The Seebeck coefficient of the cell was improved from −0.86 to −1.20 mV K−1 by the addition of β-cyclodextrin. Isothermal titration calorimetry and a theoretical investigation demonstrated that the host–guest interaction increased the Seebeck coefficient.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Abraham TJ, MacFarlane DR, Pringle JM. Seebeck coefficients in ionic liquids--prospects for thermo-electrochemical cells. Chem Commun. 2011;47:6260–2.
Abraham TJ, MacFarlane DR, Pringle JM. High Seebeck coefficient redox ionic liquid electrolytes for thermal energy harvesting. Energy Environ Sci. 2013;6:2639–45.
Abdullah N, Noor NLM, Nordin AR, Halcrow MA, MacFarlane DR, Lazar MA, Pringle JM, Bruce DW, Donnio B, Heinrich B. Spin-crossover, mesomorphic and thermoelectrical properties of cobalt(ii) complexes with alkylated N 3 -Schiff bases. J Mater Chem C. 2015;3:2491–9.
Abraham T, Tachikawa N, Macfarlane D, Pringle J. Investigation of the kinetic and mass transport limitations in thermoelectrochemical cells with different electrode materials. Phys Chem Chem Phys. 2014;16:2527–32.
MacFarlane DR, Tachikawa N, Forsyth M, Pringle JM, Howlett PC, Elliott GD, Davis JH, Watanabe M, Simon P, Angell CA. Energy applications of ionic liquids. Energy Environ Sci. 2014;7:232–50.
Lazar MA, Al-Masri D, MacFarlane DR, Pringle JM. Enhanced thermal energy harvesting performance of a cobalt redox couple in ionic liquid-solvent mixtures. Phys Chem Chem Phys. 2016;18:1404–10.
Salazar PF, Stephens ST, Kazim AH, Pringle JM, Cola BA. Enhanced thermo-electrochemical power using carbon nanotube additives in ionic liquid redox electrolytes. J Mater Chem A. 2014;2:20676–82.
Mua Y, Quickenden T. Power conversion efficiency, electrode separation, and overpotential in the ferricyanide/ferrocyanide thermogalvanic cell. J Electrochem Soc. 1996;143:2558.
Kuzminskii YV, Zasukha VA, Kuzminskaya GY. Thermoelectric effects in electrochemical systems. Nonconv thermogalvanic cells. J Power Sources. 1994;52:231–42.
Quickenden TI, Vernon CF. Thermogalvanic conversion of heat to electricity. Sol Energy. 1986;36:63–72.
Gunawan A, Lin CH, Buttry DA, Mujica V, Taylor RA, Prasher RS, Phelan PE. Liquid thermoelectrics: review of recent and limited new data of thermogalvanic cell experiments. Nanoscale Microsc Thermophys Eng. 2013;17:304–23.
Burrows B. Discharge behavior of redox thermogalvanic cells. J Electrochem Soc. 1976;123:154–9.
Ikeshoji T. Thermoelectric conversion by thin-layer thermogalvanic cells with soluble redox couples. Bull Chem Soc Jpn. 1987;60:1505–14.
Koga K, Ikeshoji T, Sugawara K. Size- and temperature-dependent structural transitions in gold nanoparticles. Phys Rev Lett. 2004;92:115507.
Anari EHB, Romano M, Teh WX, Black JJ, Jiang E, Chen J, To TQ, Panchompoo J, Aldous L. Substituted ferrocenes and iodine as synergistic thermoelectrochemical heat harvesting redox couples in ionic liquids. Chem Commun (Camb). 2016;52:745–8.
Kim T, Lee JS, Lee G, Yoon H, Yoon J, Kang TJ, Kim YH. High thermopower of ferri/ferrocyanide redox couple in organic-water solutions. Nano Energy. 2017;31:160–7.
Hu R, Cola BA, Haram N, Barisci JN, Lee S, Stoughton S, Wallace G, Too C, Thomas M, Gestos A, Dela Cruz ME, Ferraris JP, Zakhidov AA, Baughman RH. Harvesting waste thermal energy using a carbon-nanotube-based thermo-electrochemical cell. Nano Lett. 2010;10:838–46.
Zhou H, Yamada T, Kimizuka N. Supramolecular thermo-electrochemical cells: enhanced thermoelectric performance by host-guest complexation and salt-induced crystallization. J Am Chem Soc. 2016;138:10502–7.
Salazar PF, Kumar S, Cola BA. Design and optimization of thermo-electrochemical cells. J Appl Electrochem. 2014;44:325–36.
Migita T, Tachikawa N, Katayama Y, Miura T. Thermoelectromotive force of some redox couples in an amide-type room-temperature ionic liquid. Electrochemistry. 2009;77:639–41.
Xiang C, Liu C, Hao C, Wang Z, Che L, Zhou X. A self-powered acceleration sensor with flexible materials based on triboelectric effect. Nano Energy. 2017;31:469–77.
Quickenden TI, Mua Y. A review of power generation in aqueous thermogalvanic cells. J Electrochem Soc. 1995;142:3985–94.
Matsue T, Evans D, Osa T, Kobayashi N. Electron-transfer reactions associated with host-guest complexation. oxidation of ferrocenecarboxylic acid in the presence of beta-cyclodextrin. J Am Chem Soc. 1985;107:3411–7.
Acknowledgements:
This work was supported by JSPS KAKENHI Grant Numbers JP25220805, JP16H06513 (Coordination Asymmetry), 26708007 and 26600026, and by JST PRESTO Grant Number JPMJPR141D, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Yamada, T., Zou, X., Liang, Y. et al. A supramolecular thermocell consisting of ferrocenecarboxylate and β-cyclodextrin that has a negative Seebeck coefficient. Polym J 50, 771–774 (2018). https://doi.org/10.1038/s41428-018-0061-7
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41428-018-0061-7