Abstract
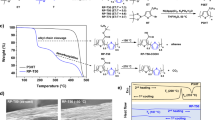
This study investigates the influence of the solvent used to prepare films of a poly(3-hexylthiophene) (P3HT) and poly(lactic acid) (PLA) blend on the morphology and charge transport mobility of field-effect transistors (FETs). Films prepared from CH2Cl2, a poor solvent for P3HT, tended to form well-defined nanowires, attributable to P3HT self-assembly via a solubility-induced process. This phenomenon resulted in a mobility of 5.30 × 10−3 cm2 (Vs)−1 and an on/off ratio of 3.23 × 103 in a CH2Cl2-solvent P3HT/PLA-blend system with a P3HT content of 10 wt%. Even a blend with 2 wt% P3HT exhibited a mobility of 1.76 × 10−3 cm2 (Vs)−1. However, in blend systems where CHCl3 solvent was employed in film preparation, the mobility decreased as the PLA content increased, and almost no electrical characteristics were exhibited at 50 wt% P3HT due to the isolated, spherical, phase-separated morphology of P3HT aggregation. Moreover, in CH2Cl2 solvent systems, the mobility of the P3HT/PLA (10/90) blend decreased from 5.3 × 10−3 cm2 (Vs)−1 (in a glove box) to 3.7 × 10−3 cm2 (Vs)−1 (after 28 days of air exposure), whereas that of 100 wt% P3HT declined by approximately one order of magnitude. These results confirm that P3HT/PLA blends prepared from CH2Cl2 solvent can be used to fabricate environmentally friendly, low-cost FETs with favorable air stability.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Lin HW, Lee WY, Lu C, Lin CJ, Wu HC, Lin YW, Ahn B, Rho Y, Ree M, Chen WC. Biaxially extended quaterthiophene-thiophene and -selenophene conjugated polymers for optoelectronic device applications. Polym Chem. 2012;3:767–77.
Lee WY, Cheng KF, Wang TF, Chueh CC, Chen WC, Tuan CS, Lin JL. Effects of acceptors on the electronic and optoelectronic properties of fluorene-based donor–acceptor–donor copolymers. Macromol Chem Phys. 2007;208:1919–27.
Zaumseil J, Sirringhaus H. Electron and ambipolar transport in organic field-effect transistors. Chem Rev. 2007;107:1296–323.
Murphy AR, Fre´chet JMJ. Organic semiconducting oligomers for use in thin film transistors. Chem Rev. 2007;107:1066–96.
Dimitrakopoulos CD, Malenfant PRL. Organic thin film transistors for large area electronics. Adv Mater. 2002;14:99–117.
Katz HE. Recent advances in semiconductor performance and printing processes for organic transistor-based electronics. Chem Mater. 2004;16:4748–56.
Lin HW, Lee WY, Chen WC. Selenophene-DPP donor–acceptor conjugated polymer for high performance ambipolar field effect transistor and nonvolatile memory applications. J Mater Chem. 2012;22:2120–8.
Wang C, Dong H, Hu W, Liu Y, Zhu D. Semiconducting pi-conjugated systems in field-effect transistors: a material odyssey of organic electronics. Chem Rev. 2012;112:2208–67.
Sirringhaus H, Brown PJ, Friend RH, Nielsen MM, Bechgaard K, Langeveld-Voss BMW, Spiering AJH, Janssen RAJ, Meijer EW, Herwig P, de Leeuw DM. Two-dimensional charge transport in self-organized, high-mobility conjugated polymers. Nature. 1999;401:685–8.
Huitema HEA, Gelinck GH, van der Putten JBPH, Kuijk KE, Hart KM, Cantatore E, de Leeuw DM. Active-matrix displays driven by solution processed polymeric transistors. Adv Mater. 2002;14:1201–4.
Janata J, Josowicz M. Conducting polymers in electronics chemical sensors. Nat Mater. 2003;2:19–24.
Dodabalapur A. Organic and polymer transistors for electronics. Mater Today. 2006;9:24–30.
Huitema HEA, Gelinck GH, J. van der Putten BPH, Kuijk KE, Hart CM, Cantatore E, Herwig PT, van Breemen AJJM, de Leeuw DM. Plastic transistors in active-matrix displays. Nature. 2001;414:598–9.
Böhm M, Ullmann A, Zipperer D, Knobloch A, Glauert WH, Fix W. Printable electronics for polymer RFID applications. IEEE Int Conf Electron Devices Solid-State Circuits. 2006;1034–41.
Kline RJ, McGehee MD, Kadnikova EN, Liu JS, Frechet JMJ. Controlling the field-effect mobility of regioregular polythiophene by changing the molecular weight. Adv Mater. 2003;15:1519–22.
Kline RJ, McGehee MD, Kadnikova EN, Liu JS, Frechet JMJ, Toney MF. Dependence of regioregular poly(3-hexylthiophene) film morphology and field-effect mobility on molecular weight. Macromolecules. 2005;38:3312–19.
Chen JY, Kuo CC, Lai CS, Chen WC, Chen HL. Manipulation on the morphology and electrical properties of aligned electrospun nanofibers of poly(3-hexylthiophene) for field-effect transistor applications. Macromolecules. 2011;44:2883–92.
Lee YH, Yen WC, Su WF, Dai CA. Self-assembly and phase transformations of π-conjugated block copolymers that bend and twist: from rigid-rod nanowires to highly curvaceous gyroids. Soft Matter. 2011;7:10429–42.
Dong H, Fu X, Liu J, Wang Z, Hu W. 25th anniversary article: key points for high-mobility organic field-effect transistors. Adv Mater. 2013;25:6158–83.
Miozzo L, Yassar A, Horowitz G. Surface engineering for high performance organic electronic devices: the chemical approach. J Mater Chem. 2010;20:2513–38.
Zhao K, Ding Z, Xue L, Han Y. Crystallization-induced phase segregation based on double-crystalline blends of poly(3-hexylthiophene) and poly(ethylene glycol)s. Macromol Rapid Commun. 2010;31:532–8.
Babel A, Jenekhe SA. Charge carrier mobility in blends of poly(9,9-dioctylfluorene) and poly(3-hexylthiophene). Macromolecules. 2003;36:7759–64.
Kumar A, Baklar MA, Scott K, Kreouzis T, Stingelin-Stutzmann N. Efficient, stable bulk charge transport in crystalline/crystalline semiconductorâ insulator blends. Adv Mater. 2009;21:4447–51.
Russell DM, Newsome CJ, Li SP, Kugler T, Ishida M, Shimoda T. Blends of semiconductor polymer and small molecular crystals for improved-performance thin-film transistors. Appl Phys Lett. 2005;87:222109.
Sparrowe D, Baklar M, Stingelin N. Low-temperature printing of crystalline:crystalline polymer blend transistors. Org Electron. 2010;11:1296–1300.
Babel A, Jenekhe SA. Field-effect mobility of charge carriers in blends of regioregular poly(3-alkylthiophene)s. J Phys Chem B. 2003;107:1749–54.
Lee WY, Giri G, Diao Y, Tassone CJ, Matthews JR, Sorensen ML, Mannsfeld SCB, Chen WC, Fong HH, Tok JBH, Toney MF, He M, Bao Z. Effect of non-chlorinated mixed solvents on charge transport and morphology of solution-processed polymer field-effect transistors. Adv Funct Mater. 2014;24:3524–34.
Nicho ME, García-Escobar CH, Arenas MC, Altuzar-Coello P, Cruz-Silva R, Güizado-Rodríguez M. Influence of P3HT concentration on morphological, optical and electrical properties of P3HT/PS and P3HT/PMMA binary blends. Mater Sci Eng B. 2011;176:1393–1400.
Qiu L, Lee WH, Wang X, Kim JS, Lim JA, Kwak D, Lee S, Cho K. Organic thin-film transistors based on polythiophene nanowires embedded in insulating polymer. Adv Mater. 2009;21:1349–53.
Lee Y, Kim JK, Chiu CH, Lan YK, Huang CI. Phase behavior of poly(3-alkylthiophene)/polystyrene blends. Polymer. 2009;50:4944–49.
Kergoat L, Battaglini N, Miozzo L, Piro B, Pham MC, Yassar A, Horowitz G. Use of poly(3-hexylthiophene)/poly(methyl methacrylate) (P3HT/PMMA) blends to improve the performance of water-gated organic field-effect transistors. Org Electron. 2011;12:1253–57.
Goffri S, Muller C, Stingelin-Stutzmann N, Breiby DW, Radano CP, Andreasen JW, Thompson R, Janssen RAJ, Nielsen MM, Smith P, Sirringhaus H. Multicomponent semiconducting polymer systems with low crystallization-induced percolation threshold. Nat Mater. 2006;5:950–6.
Babel A, Jenekhe SA. Morphology and field-effect mobility of charge carriers in binary blends of poly(3-hexylthiophene) with poly[2-methoxy-5-(2-ethylhexoxy)-1,4-phenylenevinylene] and polystyrene. Macromolecules. 2004;37:9835–40.
Lee SW, Lee HJ, Choi JH, Koh WG, Myoung JM, Hur JH, Park JJ, Cho JH, Jeong U. Periodic array of polyelectrolyte-gated organic transistors from electrospun poly(3-hexylthiophene) nanofibers. Nano Lett. 2010;10:347–51.
Qiu L, Lim JA, Wang X, Lee WH, Hwang M, Cho K. Versatile use of vertical-phase-separation-induced bilayer structures in organic thin-film transistors. Adv Mater. 2008;20:1141–5.
Lin JC, Lee WY, Wu HC, Chou CC, Chiu YC, Sun YS, Chen WC. Morphology and field-effect transistor characteristics of semicrystalline poly(3-hexylthiophene) and poly(stearyl acrylate) blend nanowires. J Mater Chem. 2012;22:14682.
Lee S, Jeon H, Jang M, Baek KY, Yang H. Tunable solubility parameter of poly(3-hexyl thiophene) with hydrophobic side-chains to achieve rubbery conjugated films. ACS Appl Mater Interfaces. 2015;7:1290–7.
Shikinami Y, Okuno M. Bioresorbable devices made of forged composites of hydroxyapatite (HA) particles and poly l-lactide (PLLA). Part II: practical properties of miniscrews and miniplates. Biomaterials. 2001;22:3197–211.
Tsuji H. In vitro hydrolysis of blends from enantiomeric poly(lactide)s. Part 4: well-homo-crystallized blend and nonblended films. Biomaterials. 2003;24:537–47.
Li S, Vert M. Synthesis, characterization, and stereocomplex-induced gelation of block copolymers prepared by ring-opening polymerization of L(D)-lactide in the presence of poly(ethylene glycol). Macromolecules. 2003;36:8008–14.
Ho V, Boudouris BW, McCulloch BL, Shuttle CG, Burkhardt M, Chabinyc ML, Segalman RA. Poly(3-alkylthiophene) diblock copolymers with ordered microstructures and continuous semiconducting pathways. J Am Chem Soc. 2011;133:9270–3.
Grancharov G, Coulembier O, Surin M, Lazzaroni R, Dubois P. Stereocomplexed materials based on poly(3-hexylthiophene)-b-poly(lactide) block copolymers: synthesis by organic catalysis, thermal properties, and microscopic morphology. Macromolecules. 2010;43:8957–64.
Smith J, Hamilton R, McCulloch I, Stingelin-Stutzmann N, Heeney M, Bradley DDC, Anthopoulos TD. Solution-processed organic transistors based on semiconducting blends. J Mater Chem. 2010;20:2562–74.
Brown PJ, Thomas DS, Köhler A, Wilson JS, Kim JS, Ramsdale CM, Sirringhaus H, Friend RH. Effect of interchain interactions on the absorption and emission of poly(3-hexylthiophene). Phys Rev B. 2003;67:064203. 1-16
Machui F, Abbott S, Waller D, Koppe M, Brabec CJ. Determination of solubility parameters for organic semiconductor formulations. Macromol Chem Phys. 2011;212:2159–65.
Thomasin C, Merkle HP, Gander B. Drug microencapsulation by PLA/PLGA coacervation in the light of thermodynamics. 2. Parameters determining microsphere formation. J Pharm Sci. 1998;87:269–75.
Ham HT, Choi YS, Chung IJ. An explanation of dispersion states of single-walled carbon nanotubes in solvents and aqueous surfactant solutions using solubility parameters. J Colloid Interface Sci. 2005;286:216–23.
Chiu MY, Jeng US, Su MS, Wei KH. Morphologies of self-organizing regioregular conjugated polymer/fullerene aggregates in thin film solar cells. Macromolecules. 2010;43:428–32.
Acknowledgements
C.-C.K. acknowledges the Ministry of Science and Technology, Taiwan, and National Taipei University of Technology and Chang Gung Memorial Hospital Joint Research Program (NTUT-CGMH-107T140-2), NTUT-CGMH Joint Research Program, for financial support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Cho, CJ., Chen, SY., Kuo, CC. et al. Morphology and optoelectronic characteristics of organic field-effect transistors based on blends of polylactic acid and poly(3-hexylthiophene). Polym J 50, 975–987 (2018). https://doi.org/10.1038/s41428-018-0087-x
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41428-018-0087-x
This article is cited by
-
Role of proton conducting polyelectrolyte on the organic photovoltaics efficiency
Journal of Materials Science: Materials in Electronics (2024)