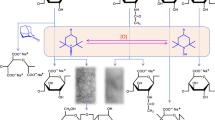
Abstract
Small-angle X-ray scattering (SAXS) and circular dichroism measurements were carried out for NaHeparin and a triple-helical peptide, H-(Pro-Pro-Gly)10-OH (PPG10), in aqueous sodium chloride (NaCl) at ionic strengths of 20, 50, and 150 mM at different temperatures. While PPG10 forms a triple helix below 25 °C, the melting temperature of the triple helix in the mixed solution is considerably higher (~10 °C) at low CS values than without NaHeparin. Part of the PPG10 molecules formed complexes with NaHeparin in 20 and 50 mM aqueous NaCl at 15 °C, but all solutes were molecularly dispersed at 75 °C, indicating that only triple helices form complexes with NaHeparin. Electrostatic attraction plays an important role in the complexation, since no complex formation was observed in 150 mM aqueous NaCl. The scattering function of the complex was explained by the presence of a thick wormlike chain, indicating that the molecular shape is different from that of the previously investigated complex with polyacrylic acid and carboxymethyl amylose. This suggests appreciable attractive interaction between the triple-helical part of PPG10 and NaHeparin.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Engel J, Kurtz J, Katchalski E, Berger A. Polymers of tripeptides as collagen models. J Mol Biol. 1966;17:255–72.
Sakakibara S, Kishida Y, Kikuchi Y, Sakai R, Kakiuchi K. Synthesis of poly-(L-prolyl-L-prolylglycyl) of defined molecular weights. Bull Chem Soc Jpn. 1968;41:1273.
Kobayashi Y, Sakai R, Kakiuchi K, Isemura T. Physicochemical analysis of (Pro-Pro-Gly)n with defined molecular weight-temperature dependence of molecular weight in aqueous solution. Biopolymers. 1970;9:415–25.
Stokke BT, Elgsaeter A, Brant DA, Kuge T, Kitamura S. Macromolecular cyclization of (1– > 6)-branched-(1– > 3)-beta-D-glucans observed after denaturation-renaturation of the triple-helical structure. Biopolymers. 1993;33:193–8.
Matsuda Y, Sugiura F, Okumura K, Tasaka S. Renaturation behavior of xanthan with high molar mass and wide molar mass distribution. Polym J. 2016;48:653–8.
Matsuda Y, Okumura K, Tasaka S. Molar mass dependence of structure of xanthan thermally denatured and renatured in dilute solution. Polym J. 2018;50:1043–9.
Shoulders MD, Raines RT. Collagen structure and stability. Annu Rev Biochem. 2009;78:929–58.
Berisio R, De Simone A, Ruggiero A, Improta R, Vitagliano L. Role of side chains in collagen triple helix stabilization and partner recognition. J Pept Sci. 2009;15:131–40.
Okuyama K. Revisiting the molecular structure of collagen. Connect Tissue Res. 2008;49:299–310.
Fields GB. Synthesis and biological applications of collagen-model triple-helical peptides. Org Biomol Chem. 2010;8:1237–58.
Fallas JA, O’Leary LER, Hartgerink JD. Synthetic collagen mimics: self-assembly of homotrimers, heterotrimers and higher order structures. Chem Soc Rev. 2010;39:3510–27.
Fan CY, Huang CC, Chiu WC, Lai CC, Liou GG, Li HC, et al. Production of multivalent protein binders using a self-trimerizing collagen-like peptide scaffold. FASEB J. 2008;22:3795–804.
Ndinguri MW, Zheleznyak A, Lauer JL, Anderson CJ, Fields GB. Application of collagen-model triple-helical peptide-amphiphiles for CD44-targeted drug delivery systems. J. Drug Deliv. 2012;2012:592602.
Yasui H, Yamazaki CM, Nose H, Awada C, Takao T, Koide T. Potential of collagen-like triple helical peptides as drug carriers: Their in vivo distribution, metabolism, and excretion profiles in rodents. Biopolymers. 2013;100:705–13.
Yamazaki CM, Nakase I, Endo H, Kishimoto S, Mashiyama Y, Masuda R, et al. Collagen-like cell-penetrating peptides. Angew Chem Int Ed. 2013;52:5497–500.
Shinde A, Feher KM, Hu C, Slowinska K. Peptide internalization enabled by folding: triple helical cell-penetrating peptides. J Pept Sci. 2015;21:77–84.
Bennink LL, Smith DJ, Foss CA, Pomper MG, Li Y, Yu SM. High serum stability of collagen hybridizing peptides and their fluorophore conjugates. Mol Pharm. 2017;14:1906–15.
Okuyama K, Okuyama K, Arnott S, Takayanagi M, Kakudo M. Crystal and molecular structure of a collagen-like polypeptide (Pro-Pro-Gly)10. J Mol Biol. 1981;152:427–43.
Terao K, Mizuno K, Murashima M, Kita Y, Hongo C, Okuyama K, et al. Chain dimensions and hydration behavior of collagen model peptides in aqueous solution: [Glycyl-4(R)-hydroxyprolyl-4(R)-hydroxyproline](n), [glycylprolyl-4(R)-hydroxyproline](n), and some related model peptides. Macromolecules. 2008;41:7203–10.
Shikata T, Minakawa A, Okuyama K. Structure, dynamics, and hydration of a collagen model polypeptide, (L-prolyl-L-prolylglycyl)10, in aqueous media: A chemical equilibrium analysis of triple helix-to-single coil transition. J Phys Chem B. 2009;113:14504–12.
Kita Y, Terao K, Sato T. Stabilization of the triple helical structure of a collagen model peptide by complexation with polyacrylic acid in methanol. Kobunshi Ronbunshu. 2010;67:686–9.
Terao K, Kanenaga R, Sato T, Mizuno K, Bächinger HP. Complex formation of collagen model peptides with polyelectrolytes and stabilization of the triple helical structure. Macromolecules. 2012;45:392–400.
Terao K, Kanenaga R, Yoshida T, Mizuno K, Bächinger HP. Temperature induced complex formation-deformation behavior of collagen model peptides and polyelectrolytes in aqueous solution. Polymer. 2015;64:8–13.
Ryoki A, Ida D, Terao K. Scattering function of semi-rigid cyclic polymers analyzed in terms of worm-like rings: cyclic amylose tris(phenylcarbamate) and cyclic amylose tris(n-butylcarbamate). Polym J. 2017;49:633–7.
Uramoto K, Takahashi R, Terao K, Sato T. Local and global conformations of flower micelles and flower necklaces formed by an amphiphilic alternating copolymer in aqueous solution. Polym J. 2016;48:863–7.
Shimizu N, Yatabe K, Nagatani Y, Saijyo S, Kosuge T, Igarashi N. Software development for analysis of small-angle X-ray scattering data. AIP Conf Proc. 2016;1741:050017.
Glatter O, Kratky O. Small angle X-ray scattering. London: Academic Press; 1982.
Yamakawa H, Yoshizaki T. Helical wormlike chains in polymer solutions. 2nd ed. Berlin, Germany: Springer; 2016.
Nakamura Y, Norisuye T. 2.02-Polymer properties in solutions. In: Krzysztof M, Martin M, editors. Polymer science: a comprehensive reference. Amsterdam: Elsevier, 2012. p. 5–32.
Burchard W, Kajiwara K. The statistics of stiff chain molecules. I. The particle scattering factor. Proc R Soc Lond, Ser A. 1970;316:185–99.
Nagasaka K, Yoshizaki T, Shimada J, Yamakawa H. More on the scattering function of helical wormlike chains. Macromolecules 1991;24:924–31.
Nakamura Y, Norisuye T. Brush-like polymers. In: Borsali R, Pecora R, editors. Soft matter characterization. Netherlands: Springer; 2008. p. 235–86.
Nakamura Y, Norisuye T. Scattering function for wormlike chains with finite thickness. J Polym Sci, Part B: Polym Phys. 2004;42:1398–407.
Pavlov G, Finet S, Tatarenko K, Korneeva E, Ebel C. Conformation of heparin studied with macromolecular hydrodynamic methods and X-ray scattering. Eur Biophys J. 2003;32:437–49.
Hayashi K, Tsutsumi K, Norisuye T, Teramoto A. Electrostatic contributions to chain stiffness and excluded-volume effects in sodium hyaluronate solutions. Polym J. 1996;28:922–8.
Khan S, Gor J, Mulloy B, Perkins SJ. Semi-rigid solution structures of heparin by constrained X-ray scattering modelling: new insight into heparin-protein complexes. J Mol Biol. 2010;395:504–21.
Johansson JA, Halthur T, Herranen M, Soderberg L, Elofsson U, Hilborn J. Build-up of collagen and hyaluronic acid polyelectrolyte multilayers. Biomacromolecules. 2005;6:1353–9.
Zhang J, Senger B, Vautier D, Picart C, Schaaf P, Voegel JC, et al. Natural polyelectrolyte films based on layer-by layer deposition of collagen and hyaluronic acid. Biomaterials. 2005;26:3353–61.
Li W, Zhao P, Lin C, Wen X, Katsanevakis E, Gero D, et al. Natural polyelectrolyte self-assembled multilayers based on collagen and alginate: stability and cytocompatibility. Biomacromolecules. 2013;14:2647–56.
Acknowledgements
We thank Prof. Takahiro Sato at Osaka University for the fruitful discussion and Dr. Noboru Ohta (SPring-8), Prof. Noriyuki Igarashi (KEK), and Prof. Nobutaka Shimizu (KEK) for the SAXS measurements. The SAXS data were acquired at the BL40B2 beamline in SPring-8 with the approval of the Japan Synchrotron Radiation Research Institute (JASRI) (Proposal Nos. 2015B1100, 2016A1053, and 2016B1088) and at the BL-10C beamline in KEK-PF under the approval of the Photon Factory Program Advisory Committee (Proposal No. 2011G557). This work was partially supported by JSPS KAKENHI Grant Nos. JP17K05884 and JP18H02020.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ishida, S., Yoshida, T. & Terao, K. Complex formation of a triple-helical peptide with sodium heparin. Polym J 51, 1181–1187 (2019). https://doi.org/10.1038/s41428-019-0234-z
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41428-019-0234-z
This article is cited by
-
Kinetics of the complex formation of silica nanoparticles with collagen
Polymer Journal (2021)