Abstract
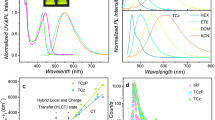
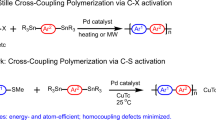
Incorporating functional groups along conjugated microporous polymer conjugated backbones (CMPs) is an effective strategy to tune optoelectronic properties and achieve superior application performance. However, due to the incompatibility of the functional groups with the polymerization reaction, introducing these functionalities into the polymeric framework is often highly challenging. Herein, we report a facile route to synthesize aldehyde-functional group flanked (and aldehyde-free) CMPs TCMP-A (and TCMP) through a Pd-catalyzed direct arylation polymerization of 1,1,2,2-tetrakis(4-bromophenyl)ethene with 2,5-di(thiophene-2-yl)terephthalaldehyde or 1,4-di(thiophen-2-yl)benzene in good yields. The methodology circumvents the preactivation of building blocks and tolerates the presence of aldehyde-functional groups during polymerization, significantly reducing the number of synthetic steps. In contrast, synthesizing these polymers via conventional coupling methods, such as Suzuki, Stille, and Sonogashira reactions, is laborious. TCMP and TCMP-A exhibit strong visible light absorption (red edge of λabs = ~605 nm) and solid-state fluorescence (λem = 620 nm; ϕf = ~5%). The pendant aldehyde-functional group in TCMP-A enables selective fluorescent chemosensing of aliphatic and aromatic amines by enhancing the fluorescence of aliphatic amines and performing fluorescence quenching for aromatic amines.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Xu Y, Jin S, Xu H, Nagai A, Jiang D. Conjugated microporous polymers: design, synthesis and application. Chem Soc Rev. 2013;42:8012–31.
Liu Q, Tang Z, Wu M, Zhou Z. Design, preparation and application of conjugated microporous polymers. Polym Int. 2014;63:381–92.
Lee JSM, Cooper AI. Advances in Conjugated Microporous Polymers. Chem Rev. 2020;120:2171–214.
Liao Y, Cheng Z, Zuo W, Thomas A, Faul CFJ. Nitrogen-Rich Conjugated Microporous Polymers: Facile Synthesis, Efficient Gas Storage, and Heterogeneous Catalysis. ACS Appl Mater Interfaces. 2017;9:38390–38400.
Zhang T, Xing G, Chen W, Chen L. Porous organic polymers: a promising platform for efficient photocatalysis. Mater Chem Front. 2020;4:332–53.
Babu HV, Bai MGM, Rao MR. Functional π-Conjugated Two-Dimensional Covalent Organic Frameworks. ACS Appl Mater Interfaces. 2019;11:11029–60.
Zhou YB, Zhan ZP. Conjugated Microporous Polymers for Heterogeneous Catalysis. Chem Asian J. 2018;13:9–19.
Dai C, Liu B. Conjugated polymers for visible-light-driven photocatalysis. Energy Environ Sci. 2020;13:24–52.
Guillerm V, Weselinski LJ, Alkordi M, Mohideen MIH, Belmabkhout Y, Cairns AJ, et al. Porous organic polymers with anchored aldehydes: a new platform for post-synthetic amine functionalization en route for enhanced CO2 adsorption properties. Chem Commun. 2014;50:1937–40.
Wisser FM, Eckhardt K, Wisser D, Böhlmann W, Grothe J, Brunner E, et al. The mechanochemical Scholl reaction as a versatile synthesis tool for the solvent-free generation of microporous polymers. Macromolecules. 2014;47:4210–6.
Wu Y, Zang Y, Xu L, Wang J, Jia H, Miao F. Synthesis of functional conjugated microporous polymer/TiO2 nanocomposites and the mechanism of the photocatalytic degradation of organic pollutants. J Mater Sci. 2021;56:7936–50.
Mu P, Sun H, Zang J, Zhu Z, Liang W, Yu F, et al. Facile tunning the morphology and porosity of a superwetting conjugated microporous polymers. React Funct Polym. 2016;106:105–11.
Liu W, Wu S, Su Q, Guo B, Ju P, Li G, et al. Difluoroborate-based conjugated organic polymer: a high-performance heterogeneous photocatalyst for oxidative coupling reactions. J Mater Sci. 2019;54:1205–12.
Ratvijitvech T, Dawson R, Laybourn A, Khimyak YZ, Adams DJ, Cooper AI. Post-synthetic modification of conjugated microporous polymers. Polymer. 2014;55:321–5.
Segura JL, Royuela S, Mar Ramos M. Post-synthetic modification of covalent organic frameworks. Chem Soc Rev. 2019;48:3903–45.
Song Y, Lan PC, Martin K, Ma S. Rational design of bifunctional conjugated microporous polymers. Nanoscale Adv. 2021;3:4891–906.
Wang ZJ, Ghasimi S, Landfester K, Zhang KAI. Photocatalytic Suzuki coupling reaction using conjugated microporous polymer with immobilized palladium nanoparticles under visible light. Chem Mater. 2015;27:1921–4.
Sun L, Zou Y, Liang Z, Yu J, Xu R. A one-pot synthetic strategy via tandem Suzuki–Heck reactions for the construction of luminescent microporous organic polymers. Polym Chem. 2013;5:471–8.
Sun L, Liang Z, Yu J, Xu R. Luminescent microporous organic polymers containing the 1,3,5-tri(4-ethenylphenyl)benzene unit constructed by Heck coupling reaction. Chem. 2013;4:1932–8.
Bergman RG. C–H activation. Nature. 2007;446:391–3.
Li J, Terec A, Yue W, Joshi H, Lu Y, Sun H, et al. π-Conjugated Discrete Oligomers Containing Planar and Nonplanar Aromatic Motifs. J Am Chem Soc. 2017;139:3089–94.
Shimizu H, Fujimoto K, Furusyo M, Maeda H, Nanai Y, Mizuno K, et al. Highly emissive π-conjugated alkynylpyrene oligomers: Their synthesis and photophysical properties. J Org Chem. 2007;72:1530–3.
Liu D-P, Chen Q, Zhao Y-C, Zhang L-M, Qi A-D, Han B-H. Fluorinated porous organic polymers via direct C-H arylation polycondensation. ACS Macro Lett. 2013;2:522–6.
Bohra H, Wang M. ACS Appl Polym Mater. Direct Arylation Polymerization for Synthesizing a Library of Conjugated Porous Polymers Containing Thiophene-Flanked Building Blocks. 2019;1:1697–706.
Huang WY, Shen ZQ, Cheng JZ, Liu LL, Yang K, Chen X, et al. C – H activation derived CPPs for photocatalytic a DMF cosolvent. J Mater Chem A. 2019;7:24222–30.
Bohra H, Li P, Yang C, Zhao Y, Wang M. “greener” and modular synthesis of triazine-based conjugated porous polymers: Via direct arylation polymerization: Structure-function relationship and photocatalytic application. Polym Chem. 2018;9:1972–82.
Hayashi S, Togawa Y, Yamamoto SI, Koizumi T, Nishi K, Asano A. Synthesis of π-conjugated network polymers based on fluoroarene and fluorescent units via direct arylation polycondensation and their porosity and fluorescent properties. J Polym Sci A Polym Chem. 2017;55:3862–7.
Efrem A, Wang K, Amaniampong PN, Yang C, Gupta S, Bohra H, et al. Direct arylation polymerization towards narrow bandgap conjugated microporous polymers with hierarchical porosity. Polym Chem. 2016;7:4862–6.
Bohra H, Tan SY, Shao J, Yang C, Efrem A, Zhao Y, et al. Narrow bandgap thienothiadiazole-based conjugated porous polymers: from facile direct arylation polymerization to tunable porosities and optoelectronic properties. Polym Chem. 2016;7:6413–21.
Hayashi S, Togawa Y, Ashida J, Nishi K, Asano A, Koizumi T. Synthesis of p -conjugated porous polymers via direct arylation of fluoroarenes with three-arm triazine. Polymer. 2016;90:187–92.
Feng LJ, Guo JW, Zhong X, Sun ZY. Fluorinated porous poly(spirobifluorene) via direct C-H arylation: Characterization, porosity, and gas uptake. J Macromol Sci A. 2014;51:604–9.
Cao Q, Yin Q, Chen Q, Dong ZB, Han BH. Fluorinated Porous Conjugated Polyporphyrins through Direct C−H Arylation Polycondensation: Preparation, Porosity, and Use as Heterogeneous Catalysts for Baeyer–Villiger Oxidation. Chem Eur J. 2017;23:9831–7.
Cheng JZ, Liu LL, Liao G, Shen ZQ, Tan ZR, Xing YQ, et al. Achieving an unprecedented hydrogen evolution rate by solvent-exfoliated CPP-based photocatalysts. J Mater Chem A. 2020;8:5890–9.
Poste AE, Grung M, Wright RF. Amines and amine-related compounds in surface waters: A review of sources, concentrations and aquatic toxicity. Sci Total Environ. 2014;481:274–9.
Pereira L, Mondal PK, Alves M. Book chapter: Pollutants in buildings, water and living organisms; 2015. p. 297–346.
Liu FX, Bi XH, Ren ZF, Zhang GH, Wang XM, Peng PA, et al. Determination of Amines Associated with Particles by Gas Chromatography-mass Spectrometry. Chin J Anal Chem. 2017;45:477–82.
Coulier L, Kaal ER, Tienstra M, Hankemeier T. Identification and quantification of (polymeric) hindered-amine light stabilizers in polymers using pyrolysis–gas chromatography–mass spectrometry and liquid chromatography–ultraviolet absorbance detection–evaporative light scattering detection. J Chromatogr A. 2005;1062:227–38.
Łabudzińska A, Gorczyńska K. Ultraviolet spectrophotometric method for the determination of aromatic amines in chemical industry waste waters. Analyst. 1994;119:1195–8.
Kannan SK, Ambrose B, Sudalaimani S, Pandiaraj M, Giribabu K, Kathiresan M. A review on chemical and electrochemical methodologies for the sensing of biogenic amines. Anal Methods. 2020;12:3438–53.
Leng PQ, Zhao FL, Yin BC, Ye BC. A novel, colorimetric method for biogenic amine detection based on arylalkylamine N-acetyltransferase. Chem Commun. 2015;51:8712–4.
Klockow JL, Hettie KS, Glass TE. Fluorescent Sensors for Amines. Supramol Chem, II. 2017;8:447–67.
Gao M, Li S, Lin Y, Geng Y, Ling X, Wang L, et al. Fluorescent Light-Up Detection of Amine Vapors Based on Aggregation-Induced Emission. ACS Sens. 2016;1:179–84.
Schwaebel T, Schäfer V, Wenz J, Coombs BA, Tolosa J, Bunz UHF. Imine formation as a simple reaction to construct copper-reactive cruciform fluorophores. J Org Chem. 2013;78:960–5.
Freudenberg J, Kumpf J, Schäfer V, Sauter E, Wörner SJ, Brödner K, et al. Water-soluble cruciforms and distyrylbenzenes: Synthesis, characterization, and pH-dependent amine-sensing properties. J Org Chem. 2013;78:4949–59.
McGrier PL, Solntsev KM, Miao S, Tolbert LM, Miranda OR, Rotello VM, et al. Hydroxycruciforms: Amine-Responsive Fluorophores. Chem Eur J. 2008;14:4503–10.
Patze C, Broedner K, Rominger F, Trapp O, Bunz UHF. Aldehyde Cruciforms: Dosimeters for Primary and Secondary Amines. Chem Eur J. 2011;17:13720–5.
Cao X, Li Y, Gao A, Yu Y, Chang X, Hei X. Sensing Organic Amines and Quantitative Monitoring of Intracellular pH Change Using a Fluorescent Self-Assembly System. ACS Appl Polym Mater. 2019;1:1485–95.
Mallya AN, Ramamurthy PC. Investigation of selective sensing of a diamine for aldehyde by experimental and simulation studies. Analyst. 2014;139:6456–66.
Fu Y, Yao J, Xu W, Fan T, He Q, Zhu D, et al. Reversible and "fingerprint" fluorescence differentiation of organic amine vapours using a single conjugated polymer probe. Polym Chem. 2015;6:2179–82.
Yu J, Zhang C. J. Fluorescent sensing for amines with a low detection limit based on conjugated porous polymers. Mater Chem C. 2020;8:16463–9.
Bai MGM, Babu HV, Lakshmi V, Rao MR. Structure–property–function relationship of fluorescent conjugated microporous polymers. Mater Chem Front. 2021;5:2506–51.
Acknowledgements
MRR thanks the science and engineering research board (SERB), India, for partially supporting this research through its early career research award scheme (ECR/2018/002285). In addition, the authors are grateful to the sophisticated central instrumentation facility (SCIF), IIT Dharwad, and its staff members for assisting us with the material characterizations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bai M. G, M., Nipate, A.B. & Rao, M.R. Selectively sensing amines through aldehyde-functional conjugated microporous organic polymers via Pd-catalyzed direct arylation. Polym J 55, 133–140 (2023). https://doi.org/10.1038/s41428-022-00736-7
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41428-022-00736-7