Abstract
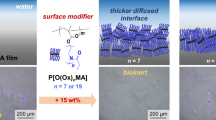
Polyrotaxane (PR) exhibits unique mechanical properties due to the ability of its cyclic molecules to move or slide along the axial chain. Thus, to design advanced polymer-based composite materials and organic devices, it is crucial to better understand the aggregation states at the surface and substrate interface in polymer films containing PR. Here, we report the depth profile of PR along the direction normal to the interface when it is mixed with polystyrene (PS). Neutron reflectivity and X-ray photoelectron spectroscopy revealed that PS and PR segregated at the surface and substrate interface, respectively, and that the extent of segregation depended on the length of PS. The surface enrichment of PS is driven by both energy and entropy, whereas the enrichment of PR at the substrate interface is energy driven.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Harada A, Li J, Kamachi M. Synthesis of a tubular polymer from threaded cyclodextrins. Nature. 1993;364:516–8.
Takata T. Polyrotaxane and polyrotaxane network: supramolecular architectures based on the concept of dynamic covalent bond chemistry. Polym J. 2006;38:1–20.
Harada A, Takashima Y, Yamaguchi H. Cyclodextrin-based supramolecular polymers. Chem Soc Rev. 2009;38:875–82.
Koyama Y. Synthesis of topologically crosslinked polymers with rotaxane-crosslinking points. Polym J. 2014;46:315–22.
Kobayashi Y. Precise synthesis of polyrotaxane and preparation of supramolecular materials based on its mobility. Polym J. 2021;53:505–13.
Javier P, Francisco M, Wayne LM. Inclusion complexes of chain molecules with cycloamyloses III. molecular dynamics simulations of polyrotaxanes formed by poly(propylene glycol) and β-cyclodextrins. Polym J. 1998;30:479–84.
Takata T, Kawasaki H, Asai S, Kihara N, Furusho Y. Radically polymerizable pseudorotaxane monomers: versatile building units for side chain. Chem Lett 1999;2:111–2.
Oku T, Furusho Y, Takata T. A concept for recyclable cross-linked polymers: topologically networked polyrotaxane capable of undergoing reversible assembly and disassembly. Angew Chem Int Ed. 2004;43:966–9.
Nakazono K, Fukasawa K, Sato T, Koyama Y, Takata T. Synthesis of acetylene-functionalized [2]rotaxane monomers directed toward side chain-type polyrotaxanes. Polym J. 2010;42:208–15.
Araki J, Kagaya K. Synthesis of polyrotaxane–glycine conjugates with various degrees of substitution via conjugation with Boc- or Z-glycine and subsequent deprotection. Polym J. 2013;45:1081–6.
Takata T, Aoki D. Topology-transformable polymers: linear–branched polymer structural transformation via the mechanical linking of polymer chains. Polym J. 2018;50:127–47.
Ito K. Novel cross-linking concept of polymer network: synthesis, structure, and properties of slide-ring gels with freely movable junctions. Polym J. 2007;39:489–99.
Yasuda Y, Hidaka Y, Mayumi K, Yamada T, Fujimoto K, Okazaki S, et al. Molecular dynamics of polyrotaxane in solution investigated by quasi-elastic neutron scattering and molecular dynamics simulation: sliding motion of rings on polymer. J Am Chem Soc. 2019;141:9655–63.
Mayumi K. Molecular dynamics and structure of polyrotaxane in solution. Polym J. 2021;53:581–6.
Bin Imran A, Esaki K, Gotoh H, Seki T, Ito K, Sakai Y, et al. Extremely stretchable thermosensitive hydrogels by introducing slide-ring polyrotaxane cross-linkers and ionic groups into the polymer network. Nat Commun. 2014;5:5124.
Tanaka A, Kato K, Ito K, Urayama K. Pronounced effects of the densities of threaded rings on the strain-dependent Poisson’s ratio of polyrotaxane gels with movable cross-links. Soft Matter. 2018;14:2808–15.
Qiu Y, Song B, Pezzato C, Shen D, Liu W, Zhang L, et al. A precise polyrotaxane synthesizer. Science. 2020;368:1247–53.
Hart LF, Hertzog JE, Rauscher PM, Rawe BW, Tranquilli MM, Rowan SJ. Material properties and applications of mechanically interlocked polymers. Nat Rev Mater. 2021;6:508–30.
Okumura Y, Ito K. The polyrotaxane gel: a topological gel figure-of-eight cross links. Adv Mater. 2001;13:485–7.
Liu C, Morimoto N, Ito K. Tough hydrogels with rapid self reinforcement. Science. 2021;372:1078–81.
Mayumi K. Tough polymer gels reinforced by strain-induced crystallization. Polym J. 2025;57:449–53.
Liu S, Hayashi T, Hara M, Seki T, Ito K, Takeoka Y. Optimal conditions for the use of polyrotaxane as a cross-linker in preparing elastomers with high toughnesses. Polym J. 2024;56:589–98.
Gotoh H, Liu C, Bin Imran A, Hara M, Seki T, Mayumi K, et al. Optically transparent, high-toughness elastomer using a polyrotaxane cross-linker as a molecular pulley. Sci Adv. 2018;4:7629–38.
Li G, Kato K, Mayumi K, Yokoyama H, Ito K. Efficient mechanical toughening of polylactic acid without substantial decreases in stiffness and transparency by the reactive grafting of polyrotaxanes. J Incl Phenom Macrocycl Chem. 2019;93:107–16.
Molero G, Liu C, Zhu Z, Chen Q, Peterson SR, Kolluru PV, et al. Fracture behavior of polyrotaxane-toughened poly(methyl methacrylate). Langmuir. 2022;38:2335–45.
Wang XS, Kim HK, Fujita Y, Sudo A, Nishida H, Endo T. Relaxation and reinforcing effects of polyrotaxane in an epoxy resin matrix. Macromolecules. 2006;39:1046–52.
Isobe Y, Sudo A, Endo T. Thermally dissociable pseudo-polyrotaxane as a supramolecular shrinkage suppressor for epoxy–amine curing system. J Polym Sci A. 2008;46:2305–8.
Pruksawan S, Samitsu S, Yokoyama H, Naito M. Homogeneously dispersed polyrotaxane in epoxy adhesive and its improvement in the fracture toughness. Macromolecules. 2019;52:2464–75.
Hanafusa A, Ando S, Ozawa S, Ito M, Hasegawa R, Mayumi K, et al. Viscoelastic relaxation attributed to the molecular dynamics of polyrotaxane confined in an epoxy resin network. Polym J. 2020;52:1211–21.
Ando S, Hirano M, Watakabe L, Yokoyama H, Ito K. Environmentally friendly sustainable thermoset vitrimer-containing polyrotaxane. ACS Mater Lett. 2023;5:3156–60.
Zhu Z, Chen H, Zhu X, Uenuma S, Ito K, Kotaki M, et al. Fracture behavior of polybenzoxazine toughened by polyrotaxane molecules and core–shell rubber. ACS Appl Eng Mater. 2023;1:1048–57.
Hanafusa A, Ando S, Yuge T, Ozawa S, Ito M, Hasegawa R, et al. Epoxy resins containing epoxy-modified polyrotaxanes. Polymer. 2023;278:126007.
Bhatia QS, Pan DH, Koberstein JT. Preferential surface adsorption in miscible blends of polystyrene and poly(vinyl methyl ether). Macromolecules. 1988;21:2166–75.
Jones RAL, Kramer EJ, Rafailovich MH, Sokolov J, Schwarz SA. Surface enrichment in an isotopic polymer blend. Phys Rev Lett 1989;62:280–3.
Hirata T, Matsuno H, Tanaka M, Tanaka K. Surface segregation of poly(2-methoxyethyl acrylate) in a mixture with poly(methyl methacrylate). Phys Chem Chem Phys. 2011;13:4928–34.
Hirata T, Matsuno H, Kawaguchi D, Yamada NL, Tanaka M, Tanaka K. Effect of interfacial structure on bioinert properties of poly(2-methoxyethyl acrylate)/poly(methyl methacrylate) blend films in water. Phys Chem Chem Phys. 2015;17:17399–405.
Russell TP, Coulon G, Deline VR, Miller DC. Characteristics of the surface-induced orientation for symmetric diblock PS/PMMA copolymers. Macromolecules. 1989;22:4600–6.
Tanaka K, Takahara A, Kajiyama T. Surface molecular motion in thin films of poly(styrene-block-methyl methacrylate) diblock copolymer. Acta Polym. 1995;46:476–82.
Hikita M, Tanaka K, Nakamura T, Kajiyama T, Takahara A. Aggregation states and surface wettability in films of poly(styrene-block-2-perfluorooctyl ethyl acrylate) diblock copolymers synthesized by atom transfer radical polymerization. Langmuir. 2004;20:5304–10.
Tanaka K, Sanada N, Hikita M, Nakamura T, Kajiyama T, Takahara A. Surface depth analysis for fluorinated block copolymer films by X-ray photoelectron spectroscopy using C60 cluster ion beam. Appl Surf Sci. 2008;254:5435–8.
Uchida K, Mita K, Yamamoto S, Tanaka K. Conformational relaxation of ethylene-propylene-diene terpolymer at a solid interface. Polym J. 2023;55:683–90.
Narumi H, Oda Y. Surface orientation of amphiphilic block copolymers via thermal annealing. Polym J. 2025;57:335–9.
Yokoyama H. New developments in polymer brush fabrication: concepts and physical properties of dynamic polymer brushes. Polym J. 2023;55:735–42.
Saito M, Ito K, Yokoyama H. Negative interfacial energies of dynamic polymer brush interfaces: a discussion of the free energy balance. Polym J. 2023;55:897–902.
Andrade JD. Surface and interfacial aspects of biomedical polymers: Volume 1 Surface chemistry and physics. New York: Plenum Press; 1985.
Sanchez IC. Physics of Polymer surfaces and interfaces. Boston: Butterworth-Heinemann; 1992.
Jones RAL, Richards RW. Polymers at surfaces and interfaces. Cambridge: Cambridge University Press; 1999.
Kajiyama T, Tanaka K, Takahara A. Surface segregation of the higher surface free energy component in symmetric polymer blend films. Macromolecules. 1998;31:3746–9.
Matsumoto T, Kannan E, Tomioka M, Nishino T. Effects of the high side-chain densities of hydrophobic poly(substituted methylene)s on their surface free energies. Polym J. 2022;54:1081–9.
Tanaka K, Kajiyama T, Takahara A, Tasaki S. A Novel method to examine surface composition in mixtures of chemically identical two polymers with different molecular weights. Macromolecules. 2002;35:4702–6.
Aoki M, Shundo A, Okamoto K, Ganbe T, Tanaka K. Segregation of an amine component in a model epoxy resin at a copper interface. Polym J. 2019;51:359–63.
Yamamoto S, Tanaka K. Entropy-driven segregation in epoxy-amine systems at a copper interface. Soft Matter. 2021;17:1359–67.
Tanaka K, Jiang X, Nakamura K, Takahara A, Kajiyama T, Ishizone T, et al. Effect of chain end chemistry on surface molecular motion of polystyrene films. Macromolecules. 1998;31:5148–9.
Satomi N, Tanaka K, Takahara A, Kajiyama T, Ishizone T, Nakahama S. Surface molecular motion of monodisperse α,ω-diamino-terminated and α,ω-dicarboxy-terminated polystyrenes. Macromolecules. 2001;34:8761–7.
Tanaka K, Kawaguchi D, Yokoe Y, Kajiyama T, Takahara A, Tasaki S. Surface segregation of chain ends in α,ω-fluoroalkyl-terminated polystyrenes films. Polymer. 2003;44:4171–7.
Kawaguchi D, Tanaka K, Kajiyama T, Takahara A, Tasaki S. Surface composition control via chain end segregation in blend films of polystyrene and poly(vinyl methyl ether). Macromolecules. 2003;36:6824–30.
Kawaguchi D, Tanaka K, Torikai N, Takahara A, Kajiyama T. Surface and interfacial segregation in blends of polystyrene with functional end groups and deuterated polystyrene. Langmuir. 2007;23:7269–75.
Matsuno H, Tsukamoto R, Shimomura S, Hirai T, Oda Y, Tanaka K. Platelet-adhesion behavior synchronized with surface rearrangement in a film of poly(methyl methacrylate) terminated with elemental blocks. Polym J. 2016;48:413–9.
Wu DT, Fredrickson GH. Effect of architecture in the surface segregation of polymer blends. Macromolecules. 1996;29:7919–30.
Yethiraj A. Integral equation theory for the surface segregation from blends of linear and star polymers. Comput Theor Polym Sci. 2000;10:115–23.
Minnikanti VS, Archer LA. Entropic attraction of polymers toward surfaces and its relationship to surface tension. Macromolecules. 2006;39:7718–28.
Sugimoto S, Oda Y, Hirata T, Matsuyama R, Matsuno H, Tanaka K. Surface segregation of a branched polymer with hydrophilic poly[2-(2-ethoxy)ethoxyethyl vinyl ether] side chains. Polym Chem. 2017;8:505–10.
Oda Y, Inutsuka M, Awane R, Totani M, Yamada NL, Haraguchi M, et al. A dynamic interface based on segregation of an amphiphilic hyperbranched polymer containing fluoroalkyl and oligo(ethylene oxide) moieties. Macromolecules. 2020;53:2380–7.
Karim A, Mansour A, Felcher GP, Russell TP. Short-time relaxation at polymer interfaces. Phys Rev B. 1990;42:6846–50.
Green PF, Kramer EJ. Matrix effects on the diffusion of long polymer chains. Macromolecules. 1986;19:1108–14.
Nelson A. Co-refinement of multiple-contrast neutron/X-ray reflectivity data using MOTOFIT. J Appl Crystallogr. 2006;39:273–6.
Hariharan A, Kumar SK, Russell TP. Reversal of the isotopic effect in the surface behavior of binary polymer blends. J Chem Phys. 1993;98:4163–73.
Acknowledgements
This work was supported by JST-Mirai Program Grant Number JPMJMI18A2, Japan.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Taguchi, M., Miyata, N., Miyazaki, T. et al. Surface and interfacial aggregation states in thin films of a polystyrene/polyrotaxane blend. Polym J 57, 737–743 (2025). https://doi.org/10.1038/s41428-025-01030-y
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41428-025-01030-y