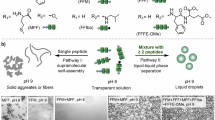
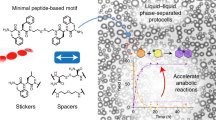
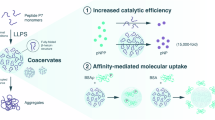
Abstract
Coacervates are condensed, liquid-like assemblies formed through liquid–liquid phase separation via associative interactions among molecular components. Owing to their membraneless nature, coacervates exhibit unique dynamic features, such as coalescence and molecular sequestration, thus serving as promising platforms for drug delivery and the regulation of biological events. In this Focus Review, representative examples of simple coacervates composed of phase-separating low-molecular-weight molecules (LMWMs) are highlighted. This review provides a minimalist design strategy for LMWM-based simple coacervates based on surfactants and peptides and summarizes their unique functions, including stimulus-responsive structural transformations. The sophisticated design of these droplets is expected to enable a wide range of applications, including studies on the origins of life, the development of artificial cells, intracellular and in vivo protein delivery, biosensing, and molecular computing.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Aumiller WM Jr, Keating CD. Experimental models for dynamic compartmentalization of biomolecules in liquid organelles: Reversible formation and partitioning in aqueous biphasic systems. Adv Colloid Interface Sci. 2017;239:75–87.
Abbas M, Lipiński WP, Wang J, Spruijt E. Peptide-based coacervates as biomimetic protocells. Chem Soc Rev. 2021;50:3690–705.
Cook AB, Novosedlik S, van Hest JCM. Complex coacervate materials as artificial cells. Acc Mater Res. 2023;4:287–98.
Gao N, Mann S. Membranized coacervate microdroplets: from versatile protocell models to cytomimetic materials. Acc Chem Res. 2023;56:297–307.
de Jong HGB, Kruyt HR. Coacervation (partial miscibility in colloid systems). Proc K Ned Akad Wet. 1929;32:849–56.
Oparin AI. The origin of life and the origin of enzymes. Adv Enzymol Relat Areas Mol Biol. 1965;27:347–80.
Strulson CA, Molden RC, Keating CD, Bevilacqua PC. RNA catalysis through compartmentalization. Nat Chem. 2012;4:941–6.
Dora Tang T-Y, Rohaida Che Hak C, Thompson AJ, Kuimova MK, Williams DS, Perriman AW, et al. Fatty acid membrane assembly on coacervate microdroplets as a step towards a hybrid protocell model. Nat Chem. 2014;6:527–33.
Qiao Y, Li M, Booth R, Mann S. Predatory behaviour in synthetic protocell communities. Nat Chem. 2017;9:110–9.
Drobot B, Iglesias-Artola JM, Le Vay K, Mayr V, Kar M, Kreysing M, et al. Compartmentalised RNA catalysis in membrane-free coacervate protocells. Nat Commun. 2018;9:3643.
Martin N, Douliez J-P, Qiao Y, Booth R, Li M, Mann S. Antagonistic chemical coupling in self-reconfigurable host-guest protocells. Nat Commun. 2018;9:3652.
Poudyal RR, Guth-Metzler RM, Veenis AJ, Frankel EA, Keating CD, Bevilacqua PC. Template-directed RNA polymerization and enhanced ribozyme catalysis inside membraneless compartments formed by coacervates. Nat Commun. 2019;10:490.
Mason AF, Yewdall NA, Welzen PLW, Shao J, van Stevendaal M, van Hest JCM, et al. Mimicking cellular compartmentalization in a hierarchical protocell through spontaneous spatial organization. ACS Cent Sci. 2019;5:1360–5.
Altenburg WJ, Yewdall NA, Vervoort DFM, van Stevendaal MHME, Mason AF, van Hest JCM. Programmed spatial organization of biomacromolecules into discrete, coacervate-based protocells. Nat Commun. 2020;11:6282.
Liu J, Tian L, Qiao Y, Zhou S, Patil AJ, Wang K, et al. Hydrogel-immobilized coacervate droplets as modular microreactor assemblies. Angew Chem Int Ed. 2020;59:6853–9.
Gobbo P, Tian L, Pavan Kumar BVVS, Turvey S, Cattelan M, Patil AJ, et al. Catalytic processing in ruthenium-based polyoxometalate coacervate protocells. Nat Commun. 2020;11:41.
Shin Y, Brangwynne CP. Liquid phase condensation in cell physiology and disease. Science. 2017;357:eaaf4382.
Banani SF, Lee HO, Hyman AA, Rosen MK. Biomolecular condensates: organizers of cellular biochemistry. Nat Rev Mol Cell Biol. 2017;18:285–98.
Alberti S, Hyman AA. Biomolecular condensates at the nexus of cellular stress, protein aggregation disease and ageing. Nat Rev Mol Cell Biol. 2021;22:196–213.
Lafontaine DLJ, Riback JA, Bascetin R, Brangwynne CP. The nucleolus as a multiphase liquid condensate. Nat Rev Mol Cell Biol. 2021;22:165–82.
Frenkel-Pinter M, Samanta M, Ashkenasy G, Leman LJ. Prebiotic peptides: molecular hubs in the origin of life. Chem Rev. 2020;120:4707–65.
Koga S, Williams DS, Perriman AW, Mann S. Peptide-nucleotide microdroplets as a step towards a membrane-free protocell model. Nat Chem. 2011;3:720–4.
Mason AF, Buddingh’ BC, Williams DS, van Hest JCM. Hierarchical self-assembly of a copolymer-stabilized coacervate protocell. J Am Chem Soc. 2017;139:17309–12.
Douliez J-P, Martin N, Gaillard C, Beneyton T, Baret J-C, Mann S, et al. Catanionic coacervate droplets as a surfactant-based membrane-free protocell model. Angew Chem Int Ed. 2017;56:13689–93.
Tian L, Li M, Patil AJ, Drinkwater BW, Mann S. Artificial morphogen-mediated differentiation in synthetic protocells. Nat Commun. 2019;10:3321.
Donau C, Späth F, Sosson M, Kriebisch BAK, Schnitter F, Tena-Solsona M, et al. Active coacervate droplets as a model for membraneless organelles and protocells. Nat Commun. 2020;11:5167.
Bhattacharya A, Niederholtmeyer H, Podolsky KA, Bhattacharya R, Song J-J, Brea RJ, et al. Lipid sponge droplets as programmable synthetic organelles. Proc Natl Acad Sci Usa. 2020;117:18206–15.
Matsuo M, Kurihara K. Proliferating coacervate droplets as the missing link between chemistry and biology in the origins of life. Nat Commun. 2021;12:5487.
Ianeselli A, Tetiker D, Stein J, Kühnlein A, Mast CB, Braun D, et al. Non-equilibrium conditions inside rock pores drive fission, maintenance and selection of coacervate protocells. Nat Chem. 2022;14:32–39.
Akishiba M, Takeuchi T, Kawaguchi Y, Sakamoto K, Yu H-H, Nakase I, et al. Cytosolic antibody delivery by lipid-sensitive endosomolytic peptide. Nat Chem. 2017;9:751–61.
Liu S, Zhang Y, Li M, Xiong L, Zhang Z, Yang X, et al. Enzyme-mediated nitric oxide production in vasoactive erythrocyte membrane-enclosed coacervate protocells. Nat Chem. 2020;12:1165–73.
Iwata T, Hirose H, Sakamoto K, Hirai Y, Arafiles JVV, Akishiba M, et al. Liquid droplet formation and facile cytosolic translocation of IgG in the presence of attenuated cationic amphiphilic lytic peptides. Angew Chem Int Ed. 2021;60:19804–12.
Bracha D, Walls MT, Brangwynne CP. Probing and engineering liquid-phase organelles. Nat Biotechnol. 2019;37:1435–45.
Shin Y, Berry J, Pannucci N, Haataja MP, Toettcher JE, Brangwynne CP. Spatiotemporal control of intracellular phase transitions using light-activated optoDroplets. Cell. 2017;168:159–71.e14.
Nakamura H, Lee AA, Afshar AS, Watanabe S, Rho E, Razavi S, et al. Intracellular production of hydrogels and synthetic RNA granules by multivalent molecular interactions. Nat Mater. 2018;17:79–89.
Dine E, Gil AA, Uribe G, Brangwynne CP, Toettcher JE. Protein phase separation provides long-term memory of transient spatial stimuli. Cell Syst. 2018;6:655–63.e5.
Reinkemeier CD, Girona GE, Lemke EA. Designer membraneless organelles enable codon reassignment of selected mRNAs in eukaryotes. Science 2019;363:eaaw2644.
Kim NY, Lee S, Yu J, Kim N, Won SS, Park H, et al. Optogenetic control of mRNA localization and translation in live cells. Nat Cell Biol. 2020;22:341–52.
Garabedian MV, Wang W, Dabdoub JB, Tong M, Caldwell RM, Benman W, et al. Designer membraneless organelles sequester native factors for control of cell behavior. Nat Chem Biol. 2021;17:998–1007.
Yoshikawa M, Yoshii T, Ikuta M, Tsukiji S. Synthetic protein condensates that inducibly recruit and release protein activity in living cells. J Am Chem Soc. 2021;143:6434–46.
Helfrich MR, Mangeney-Slavin LK, Long MS, Djoko KY, Keating CD. Aqueous phase separation in giant vesicles. J Am Chem Soc. 2002;124:13374–5.
Long MS, Jones CD, Helfrich MR, Mangeney-Slavin LK, Keating CD. Dynamic microcompartmentation in synthetic cells. Proc Natl Acad Sci Usa. 2005;102:5920–5.
Obayashi H, Wakabayashi R, Kamiya N, Goto M. Supramolecular localization in liquid-liquid phase separation and protein recruitment in confined droplets. Chem Commun. 2023;59:414–7.
Quiroz FG, Chilkoti A. Sequence heuristics to encode phase behaviour in intrinsically disordered protein polymers. Nat Mater. 2015;14:1164–71.
Wei W, Petrone L, Tan Y, Cai H, Israelachvili JN, Miserez A, et al. An underwater surface-drying peptide inspired by a mussel adhesive protein. Adv Funct Mater. 2016;26:3496–507.
Jia TZ, Chandru K, Hongo Y, Afrin R, Usui T, Myojo K, et al. Membraneless polyester microdroplets as primordial compartments at the origins of life. Proc Natl Acad Sci Usa. 2019;116:15830–5.
Gabryelczyk B, Cai H, Shi X, Sun Y, Swinkels PJM, Salentinig S, et al. Hydrogen bond guidance and aromatic stacking drive liquid-liquid phase separation of intrinsically disordered histidine-rich peptides. Nat Commun. 2019;10:5465.
Kuroyanagi S, Shimada N, Fujii S, Furuta T, Harada A, Sakurai K, et al. Highly ordered polypeptide with UCST phase separation behavior. J Am Chem Soc. 2019;141:1261–8.
Dzuricky M, Rogers BA, Shahid A, Cremer PS, Chilkoti A. De novo engineering of intracellular condensates using artificial disordered proteins. Nat Chem. 2020;12:814–25.
Scott WA, Gharakhanian EG, Bell AG, Evans D, Barun E, Houk KN, et al. Active controlled and tunable coacervation using side-chain functional α-helical homopolypeptides. J Am Chem Soc. 2021;143:18196–203.
Sun Y, Lau SY, Lim ZW, Chang SC, Ghadessy F, Partridge A, et al. Phase-separating peptides for direct cytosolic delivery and redox-activated release of macromolecular therapeutics. Nat Chem. 2022;14:274–83.
Niu J, Qiu C, Abbott NL, Gellman SH. Formation of versus recruitment to RNA-rich condensates: controlling effects exerted by peptide side chain identity. J Am Chem Soc. 2022;144:10386–95.
Deepankumar K, Guo Q, Mohanram H, Lim J, Mu Y, Pervushin K, et al. Liquid-liquid phase separation of the green mussel adhesive protein Pvfp-5 is regulated by the post-translated dopa amino acid. Adv Mater. 2022;34:e2103828.
Sun Y, Wu X, Li J, Radiom M, Mezzenga R, Verma CS, et al. Phase-separating peptide coacervates with programmable material properties for universal intracellular delivery of macromolecules. Nat Commun. 2024;15:10094.
Fares HM, Marras AE, Ting JM, Tirrell MV, Keating CD. Impact of wet-dry cycling on the phase behavior and compartmentalization properties of complex coacervates. Nat Commun. 2020;11:5423.
Wibowo A, Osada K, Matsuda H, Anraku Y, Hirose H, Kishimura A, et al. Morphology control in water of polyion complex nanoarchitectures of double-hydrophilic charged block copolymers through composition tuning and thermal treatment. Macromolecules. 2014;47:3086–92.
Sing CE, Perry SL. Recent progress in the science of complex coacervation. Soft Matter. 2020;16:2885–914.
Kaur T, Raju M, Alshareedah I, Davis RB, Potoyan DA, Banerjee PR. Sequence-encoded and composition-dependent protein-RNA interactions control multiphasic condensate morphologies. Nat Commun. 2021;12:872.
Tsuruta M, Torii T, Kohata K, Kawauchi K, Tateishi-Karimata H, Sugimoto N, et al. Controlling liquid-liquid phase separation of G-quadruplex-forming RNAs in a sequence-specific manner. Chem Commun. 2022;58:12931–4.
Wollny D, Vernot B, Wang J, Hondele M, Safrastyan A, Aron F, et al. Characterization of RNA content in individual phase-separated coacervate microdroplets. Nat Commun. 2022;13:2626.
Simon JR, Carroll NJ, Rubinstein M, Chilkoti A, López GP. Programming molecular self-assembly of intrinsically disordered proteins containing sequences of low complexity. Nat Chem. 2017;9:509–15.
Deshpande S, Brandenburg F, Lau A, Last MGF, Spoelstra WK, Reese L, et al. Spatiotemporal control of coacervate formation within liposomes. Nat Commun. 2019;10:1800.
Linsenmeier M, Kopp MRG, Grigolato F, Emmanoulidis L, Liu D, Zürcher D, et al. Dynamics of synthetic membraneless organelles in microfluidic droplets. Angew Chem Int Ed. 2019;58:14489–94.
Sato Y, Sakamoto T, Takinoue M. Sequence-based engineering of dynamic functions of micrometer-sized DNA droplets. Sci Adv. 2020;6:eaba3471.
Mountain GA, Keating CD. Formation of multiphase complex coacervates and partitioning of biomolecules within them. Biomacromolecules. 2020;21:630–40.
Lu T, Spruijt E. Multiphase complex coacervate droplets. J Am Chem Soc. 2020;142:2905–14.
Fisher RS, Elbaum-Garfinkle S. Tunable multiphase dynamics of arginine and lysine liquid condensates. Nat Commun. 2020;11:4628.
Chen Y, Yuan M, Zhang Y, Liu S, Yang X, Wang K, et al. Construction of coacervate-in-coacervate multi-compartment protocells for spatial organization of enzymatic reactions. Chem Sci. 2020;11:8617–25.
Mu W, Ji Z, Zhou M, Wu J, Lin Y, Qiao Y. Membrane-confined liquid-liquid phase separation toward artificial organelles. Sci Adv. 2021;7:eabf9000.
Karoui H, Seck MJ, Martin N. Self-programmed enzyme phase separation and multiphase coacervate droplet organization. Chem Sci. 2021;12:2794–802.
Lu T, Liese S, Schoenmakers L, Weber CA, Suzuki H, Huck WTS, et al. Endocytosis of coacervates into liposomes. J Am Chem Soc. 2022;144:13451–5.
Gong J, Tsumura N, Sato Y, Takinoue M. Computational DNA droplets recognizing miRNA sequence inputs based on liquid–liquid phase separation. Adv Funct Mater. 2022;32:2202322.
Gao N, Xu C, Yin Z, Li M, Mann S. Triggerable protocell capture in nanoparticle-caged coacervate microdroplets. J Am Chem Soc. 2022;144:3855–62.
Donau C, Späth F, Stasi M, Bergmann AM, Boekhoven J. Phase transitions in chemically fueled, multiphase complex coacervate droplets. Angew Chem Int Ed. 2022:61;e202211905.
Iglesias-Artola JM, Drobot B, Kar M, Fritsch AW, Mutschler H, Dora Tang T-Y, et al. Charge-density reduction promotes ribozyme activity in RNA-peptide coacervates via RNA fluidization and magnesium partitioning. Nat Chem. 2022;14:407–16.
Choi S, Meyer MO, Bevilacqua PC, Keating CD. Phase-specific RNA accumulation and duplex thermodynamics in multiphase coacervate models for membraneless organelles. Nat Chem. 2022;14:1110–7.
Jain A, Kassem S, Fisher RS, Wang B, Li T-D, Wang T, et al. Connected peptide modules enable controlled co-existence of self-assembled fibers inside liquid condensates. J Am Chem Soc. 2022;144:15002–7.
Xu C, Martin N, Li M, Mann S. Living material assembly of bacteriogenic protocells. Nature. 2022;609:1029–37.
Erkamp NA, Sneideris T, Ausserwöger H, Qian D, Qamar S, Nixon-Abell J, et al. Spatially non-uniform condensates emerge from dynamically arrested phase separation. Nat Commun. 2023;14:684.
Oana H, Kishimura A, Yonehara K, Yamasaki Y, Washizu M, Kataoka K. Spontaneous formation of giant unilamellar vesicles from microdroplets of a polyion complex by thermally induced phase separation. Angew Chem Int Ed. 2009;48:4613–6.
Higashiguchi K, Taira G, Kitai J-I, Hirose T, Matsuda K. Photoinduced macroscopic morphological transformation of an amphiphilic diarylethene assembly: reversible dynamic motion. J Am Chem Soc. 2015;137:2722–9.
Aumiller WM Jr, Keating CD. Phosphorylation-mediated RNA/peptide complex coacervation as a model for intracellular liquid organelles. Nat Chem. 2016;8:129–37.
Garenne D, Beven L, Navailles L, Nallet F, Dufourc EJ, Douliez J-P. Sequestration of proteins by fatty acid coacervates for their encapsulation within vesicles. Angew Chem Int Ed. 2016;55:13475–9.
Nakashima KK, Baaij JF, Spruijt E. Reversible generation of coacervate droplets in an enzymatic network. Soft Matter. 2018;14:361–7.
Nishida K, Tamura A, Yui N. pH-Responsive coacervate droplets formed from acid-labile methylated polyrotaxanes as an injectable protein carrier. Biomacromolecules. 2018;19:2238–47.
Ianiro A, Wu H, van Rijt MMJ, Vena MP, Keizer ADA, Esteves ACC, et al. Liquid-liquid phase separation during amphiphilic self-assembly. Nat Chem. 2019;11:320–8.
Jang Y, Hsieh M-C, Dautel D, Guo S, Grover MA, Champion JA. Understanding the coacervate-to-vesicle transition of globular fusion proteins to engineer protein vesicle size and membrane heterogeneity. Biomacromolecules. 2019;20:3494–503.
Love C, Steinkühler J, Gonzales DT, Yandrapalli N, Robinson T, Dimova R, et al. Reversible pH-responsive coacervate formation in lipid vesicles activates dormant enzymatic reactions. Angew Chem Int Ed. 2020;59:5950–7.
Alshareedah I, Moosa MM, Raju M, Potoyan DA, Banerjee PR. Phase transition of RNA−protein complexes into ordered hollow condensates. Proc Natl Acad Sci USA. 2020;117:15650–8.
Nakashima KK, van Haren MHI, André AAM, Robu I, Spruijt E. Active coacervate droplets are protocells that grow and resist Ostwald ripening. Nat Commun. 2021;12:3819.
Abbas M, Lipiński WP, Nakashima KK, Huck WTS, Spruijt E. A short peptide synthon for liquid-liquid phase separation. Nat Chem. 2021;13:1046–54.
Späth F, Donau C, Bergmann AM, Kränzlein M, Synatschke CV, Rieger B, et al. Molecular design of chemically fueled peptide-polyelectrolyte coacervate-based assemblies. J Am Chem Soc. 2021;143:4782–9.
Dautel DR, Champion JA. Protein vesicles self-assembled from functional globular proteins with different charge and size. Biomacromolecules. 2021;22:116–25.
Bergmann AM, Donau C, Späth F, Jahnke K, Göpfrich K, Boekhoven J. Evolution and single-droplet analysis of fuel-driven compartments by droplet-based microfluidics. Angew Chem Int Ed. 2022;61:e202203928.
Du X, Zhou J, Shi J, Xu B. Supramolecular hydrogelators and hydrogels: from soft matter to molecular biomaterials. Chem Rev. 2015;115:13165–307.
Panja S, Adams DJ. Stimuli responsive dynamic transformations in supramolecular gels. Chem Soc Rev. 2021;50:5165–5200.
Kubota R, Hamachi I. Cell-like synthetic supramolecular soft materials realized in multicomponent, non-/out-of-equilibrium dynamic systems. Adv Sci. 2023;11:e2306830.
Kubota R. Supramolecular–polymer composite hydrogels: from in situ network observation to functional properties. Bull Chem Soc Jpn. 2023;96:802–12.
Cohen I, Hiskey CF, Oster G. Critical phenomena in aqueous solutions of long-chain quaternary ammonium salts. J Colloid Sci. 1954;9:243–53.
Cohen I, Vassiliades T. Critical phenomena in aqueous solutions of long chain quaternary ammonium salts. II. Specificity and light scattering properties. J Phys Chem. 1961;65:1774–81.
Jho Y, Yoo HY, Lin Y, Han S, Hwang DS. Molecular and structural basis of low interfacial energy of complex coacervates in water. Adv Colloid Interface Sci. 2017;239:61–73.
Strey R, Jahn W, Porte G, Bassereau P. Freeze fracture electron microscopy of dilute lamellar and anomalous isotropic (L3) phases. Langmuir. 1990;6:1635–9.
Hoffmann H, Thunig C, Munkert U, Meyer HW, Richter W. From vesicles to the L3 (sponge) phase in alkyldimethylamine oxide/heptanol systems. Langmuir. 1992;8:2629–38.
Wydro MJ, Warr GG, Atkin R. Amplitude-modulated atomic force microscopy reveals the near surface nanostructure of surfactant sponge (L3) and lamellar (Lα) phases. Langmuir. 2015;31:5513–20.
Kim S, Huang J, Lee Y, Dutta S, Yoo HY, Jung YM, et al. Complexation and coacervation of like-charged polyelectrolytes inspired by mussels. Proc Natl Acad Sci USA. 2016;113:E847–53.
Huang K-Y, Yoo HY, Jho Y, Han S, Hwang DS. Bicontinuous fluid structure with low cohesive energy: molecular basis for exceptionally low interfacial tension of complex coacervate fluids. ACS Nano. 2016;10:5051–62.
Manoj Lalwani S, Eneh CI, Lutkenhaus JL. Emerging trends in the dynamics of polyelectrolyte complexes. Phys Chem Chem Phys. 2020;22:24157–77.
Peresypkin AV, Menger FM. Zwitterionic geminis. coacervate formation from a single organic compound. Org Lett. 1999;1:1347–50.
Menger FM, Peresypkin AV, Caran KL, Apkarian RP. A sponge morphology in an elementary coacervate. Langmuir. 2000;16:9113–6.
Menger FM, Peresypkin AV. A combinatorially-derived structural phase diagram for 42 zwitterionic geminis. J Am Chem Soc. 2001;123:5614–5.
Imura T, Yanagishita H, Kitamoto D. Coacervate formation from natural glycolipid: one acetyl group on the headgroup triggers coacervate-to-vesicle transition. J Am Chem Soc. 2004;126:10804–5.
Yotsuji H, Higashiguchi K, Sato R, Shigeta Y, Matsuda K. Phototransformative supramolecular assembly of amphiphilic diarylethenes realized by a combination of photochromism and lower critical solution temperature behavior. Chem Eur J. 2017;23:15059–66.
Nojima T, Iyoda T. Water-rich fluid material containing orderly condensed proteins. Angew Chem Int Ed. 2017;56:1308–12.
Nojima T, Iyoda T. Egg white-based strong hydrogel via ordered protein condensation. NPG Asia Mater. 2018;10:e460.
Shigemitsu H, Hamachi I. Design strategies of stimuli-responsive supramolecular hydrogels relying on structural analyses and cell-mimicking approaches. Acc Chem Res. 2017;50:740–50.
Sheehan F, Sementa D, Jain A, Kumar M, Tayarani-Najjaran M, Kroiss D, et al. Peptide-based supramolecular systems chemistry. Chem Rev. 2021;121:13869–914.
Reches M, Gazit E. Casting metal nanowires within discrete self-assembled peptide nanotubes. Science. 2003;300:625–7.
Yang Z, Liang G, Wang L, Xu B. Using a kinase/phosphatase switch to regulate a supramolecular hydrogel and forming the supramolecular hydrogel in vivo. J Am Chem Soc. 2006;128:3038–43.
Ikeda M, Tanida T, Yoshii T, Hamachi I. Rational molecular design of stimulus-responsive supramolecular hydrogels based on dipeptides. Adv Mater. 2011;23:2819–22.
Ikeda M, Tanida T, Yoshii T, Kurotani K, Onogi S, Urayama K, et al. Installing logic-gate responses to a variety of biological substances in supramolecular hydrogel-enzyme hybrids. Nat Chem. 2014;6:511–8.
Onogi S, Shigemitsu H, Yoshii T, Tanida T, Ikeda M, Kubota R, et al. In situ real-time imaging of self-sorted supramolecular nanofibres. Nat Chem. 2016;8:743–52.
Yuan C, Levin A, Chen W, Xing R, Zou Q, Herling TW, et al. Nucleation and growth of amino acid and peptide supramolecular polymers through liquid-liquid phase separation. Angew Chem Int Ed. 2019;58:18116–23.
Shen Y, Ruggeri FS, Vigolo D, Kamada A, Qamar S, Levin A, et al. Biomolecular condensates undergo a generic shear-mediated liquid-to-solid transition. Nat Nanotechnol. 2020;15:841–7.
Tang Y, Bera S, Yao Y, Zeng J, Lao Z, Dong X, et al. Prediction and characterization of liquid-liquid phase separation of minimalistic peptides. Cell Rep. Phys Sci. 2021;2:100579.
Joseph JA, Reinhardt A, Aguirre A, Chew PY, Russell KO, Espinosa JR, et al. Physics-driven coarse-grained model for biomolecular phase separation with near-quantitative accuracy. Nat Comput Sci. 2021;1:732–43.
Adachi K, Kawaguchi K. Predicting heteropolymer interactions: demixing and hypermixing of disordered protein sequences. Phys Rev X. 2024;14:031011.
Murthy AC, Dignon GL, Kan Y, Zerze GH, Parekh SH, Mittal J, et al. Molecular interactions underlying liquid-liquid phase separation of the FUS low-complexity domain. Nat Struct Mol Biol. 2019;26:637–48.
Martin EW, Holehouse AS, Peran I, Farag M, Incicco JJ, Bremer A, et al. Valence and patterning of aromatic residues determine the phase behavior of prion-like domains. Science. 2020;367:694–9.
Abbas M, Law JO, Grellscheid SN, Huck WTS, Spruijt E. Peptide-based coacervate-core vesicles with semipermeable membranes. Adv. Mater. 2022;34:e2202913.
Bao Y, Chen H, Xu Z, Gao J, Jiang L, Xia J. Photo-responsive phase-separating fluorescent molecules for intracellular protein delivery. Angew Chem Int Ed. 2023;62:e202307045.
Bao Y, Xu Z, Cheng K, Li X, Chen F, Yuan D, et al. Staudinger reaction-responsive coacervates for cytosolic antibody delivery and TRIM21-mediated protein degradation. J Am Chem Soc. 2025;147:3830–9.
Ado G, Noda N, Vu HT, Perron A, Mahapatra AD, Arista KP, et al. Discovery of a phase-separating small molecule that selectively sequesters tubulin in cells. Chem Sci. 2022;13:5760–6.
Kubota R, Torigoe S, Hamachi I. Temporal stimulus patterns drive differentiation of a synthetic dipeptide-based coacervate. J Am Chem Soc. 2022;144:15155–64.
Zhang H, Elbaum-Garfinkle S, Langdon EM, Taylor N, Occhipinti P, Bridges AA, et al. RNA controls polyQ protein phase transitions. Mol Cell. 2015;60:220–30.
Folkmann AW, Putnam A, Lee CF, Seydoux G. Regulation of biomolecular condensates by interfacial protein clusters. Science. 2021;373:1218–24.
Blocher M, Liu D, Luisi PL. Liposome-assisted selective polycondensation of α-amino acids and peptides: the case of charged liposomes. Macromolecules. 2000;33:5787–96.
Izgu EC, Björkbom A, Kamat NP, Lelyveld VS, Zhang W, Jia TZ, et al. N-Carboxyanhydride-mediated fatty acylation of amino acids and peptides for functionalization of protocell membranes. J Am Chem Soc. 2016;138:16669–76.
Kholodenko BN, Hancock JF, Kolch W. Signalling ballet in space and time. Nat Rev Mol Cell Biol. 2010;11:414–26.
Purvis JE, Lahav G. Encoding and decoding cellular information through signaling dynamics. Cell. 2013;152:945–56.
Cai L, Dalal CK, Elowitz MB. Frequency-modulated nuclear localization bursts coordinate gene regulation. Nature. 2008;455:485–90.
Ashall L, Horton CA, Nelson DE, Paszek P, Harper CV, Sillitoe K, et al. Pulsatile stimulation determines timing and specificity of NF-κB-dependent transcription. Science. 2009;324:242–6.
Imayoshi I, Isomura A, Harima Y, Kawaguchi K, Kori H, Miyachi H, et al. Oscillatory control of factors determining multipotency and fate in mouse neural progenitors. Science. 2013;342:1203–8.
Teders M, Pogodaev AA, Bojanov G, Huck WTS. Reversible photoswitchable inhibitors generate ultrasensitivity in out-of-equilibrium enzymatic reactions. J Am Chem Soc. 2021;143:5709–16.
Kubota R, Hiroi T, Ikuta Y, Liu Y, Hamachi I. Visualizing formation and dynamics of a three-dimensional sponge-like network of a coacervate in real time. J Am Chem Soc. 2023;145:18316–28.
Feric M, Vaidya N, Harmon TS, Mitrea DM, Zhu L, Richardson TM, et al. Coexisting liquid phases underlie nucleolar subcompartments. Cell. 2016;165:1686–97.
Jain S, Wheeler JR, Walters RW, Agrawal A, Barsic A, Parker R. ATPase-modulated stress granules contain a diverse proteome and substructure. Cell. 2016;164:487–98.
Acknowledgements
This work was supported by the JST FOREST Program (Grant Number JPMJFR2328, Japan) and by a Grant-in-Aid for Scientific Research (B) (JSPS KAKENHI Grant JP22H02195, Japan).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kubota, R. A minimalist design approach to simple coacervates from low-molecular-weight components. Polym J 57, 815–829 (2025). https://doi.org/10.1038/s41428-025-01037-5
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41428-025-01037-5