Abstract
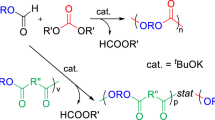
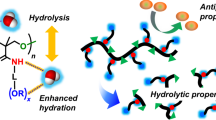
Poly(trimethylene carbonate) (PTMC) is a biodegradable polymer that is widely utilized in biomedical fields because of the soft mechanical properties conferred by its low glass transition temperature. However, its application is limited by the lack of reactive sites on its backbone for chemical modifications. To overcome this limitation, a copolymer, poly(trimethylene carbonate-r-5-methylene-1,3-dioxane-2-one) (PTMC-r-PexTMC), was developed as a platform for postpolymerization functionalization. Using thiol-ene click chemistry, diol and carboxylic acid groups were grafted onto PTMC-r-PexTMC at levels ranging from 1 to 30 mol% to tailor its hydrophilicity and surface compatibility. Thermogravimetric and contact angle analyses revealed that both the thermal stability and hydrophilicity increased with the degree of functionalization. Importantly, copolymers containing 30 mol% diol units inhibited platelet adhesion more effectively than those with equivalent carboxylic acid content did, indicating that both hydrophilicity and surface charge influence biocompatibility. Further studies involving degradation in PBS and doxorubicin release confirmed the potential of these functional groups to modulate polymer performance. These findings highlight the value of surface modification in increasing the biocompatibility of polycarbonates for advanced medical applications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Yu W, Maynard E, Chiaradia V, Arno MC, Dove AP. Aliphatic Polycarbonates from Cyclic Carbonate Monomers and Their Application as Biomaterials. Chem Rev. 2021;121:10865–10907.
Ansari I, Singh P, Mittal A, Mahato RI, Chitkara D. 2,2-Bis(Hydroxymethyl) propionic acid based cyclic carbonate monomers and their (co)polymers as advanced materials for biomedical applications. Biomaterials. 2021;275:120953.
Zhang Z, Kuijer R, Bulstra SK, Grijpma DW, Feijen J. The in vivo and in vitro degradation behavior of poly(trimethylene carbonate). Biomaterials. 2006;27:1741–8.
Amsden B. In vivo degradation mechanisms of aliphatic polycarbonates and functionalized aliphatic polycarbonates. Macromol Biosci. 2021;21:2100085.
Wu L, Wang Y, Zhao X, Mao H, Gu Z. Investigating the biodegradation mechanism of poly(trimethylene carbonate): macrophage-mediated erosion by secreting lipase. Biomacromolecules. 2023;24:921–8.
Nemoto N, Sanda F, Endo T. Cationic ring-opening polymerization of six-membered cyclic carbonates with ester groups. J Polym Sci Part A. 2001;39:1305–17.
Nederberg F, Lohmeijer BGG, Leibfarth F, Pratt RC, Choi J, Dove AP, et al. Organocatalytic ring opening polymerization of trimethylene carbonate. Biomacromolecules. 2007;8:153–60.
Saito T, Aizawa Y, Yamamoto T, Tajima K, Isono T, Satoh T. Alkali metal carboxylate as an efficient and simple catalyst for ring-opening polymerization of cyclic esters. Macromolecules. 2018;51:689–96.
Fukushima K. Poly(trimethylene carbonate)-based polymers engineered for biodegradable functional biomaterials. Biomater Sci. 2016;1:9–24.
Pratt RC, Nederberg F, Waymouth RM, Hedrick JL. Tagging alcohols with cyclic carbonate: a versatile equivalent of (meth)acrylate for ring-opening polymerization. Chem Commun. 2008;1:114–6.
Sanders DP, Fukushima K, Coady DJ, Nelson A, Fujiwara M, Yasumoto M, et al. A simple and efficient synthesis of functionalized cyclic carbonate monomers using a versatile pentafluorophenyl ester intermediate. J Am Chem Soc. 2010;132:14724–6.
Nobuoka H, Ajiro H. Novel synthesis method of ester free trimethylene carbonate derivatives. Tetrahedron Lett. 2019;60:164–70.
Watanabe Y, Takaoka S, Haga Y, Kishi K, Hakozaki S, Narumi A, et al. Organic carboxylate salt-enabled alternative synthetic routes for bio-functional cyclic carbonates and aliphatic polycarbonates. Polym Chem. 2022;13:5193–9.
Lin B, Hedrick JL, Park NH, Waymouth RM. Programmable high-throughput platform for the rapid and scalable synthesis of polyester and polycarbonate libraries. J Am Chem Soc. 2019;141:8921–7.
Tan EWP, Hedrick JL, Arrechea PL, Erdmann T, Kiyek V, Lottier S, et al. Overcoming barriers in polycarbonate synthesis: a streamlined approach for the synthesis of cyclic carbonate monomers. Macromolecules. 2021;54:1767–74.
Houw Z, Chen S, Hu W, Guo J, Li P, Hu J, et al. Long-term in vivo degradation behavior of poly(trimethlyene carbonate-co-2,2’-dimethyltrimethylene carbonate). Eur Polym J. 2022;177:111442.
Fukushima K, Watanabe Y, Ueda T, Nakai S, Kato T. Organocatalytic depolymerization of poly(trimethylene carbonate). J Polym Sci. 2022;60:3489–3500.
Fukushima K, Hakozaki S, Lang R, Haga Y, Narumi A, Tanaka M, et al. Hydrolyzable biocompatible aliphatic polycarbonates with ether-functionalized side chains attached via amide linkers. Polym J. 2024;56:431–42.
Qiu FY, Zhang M, Du FS, Li ZC. Oxidation degradable aliphatic polycarbonates with pendent phenylboronic ester. Macromolecules. 2017;50:23–34.
Prinse M, Qi R, Amsden BG. Polymer micelles for the protection and delivery of specialized pro-resolving mediators. Eur J Pharm Biopharm. 2023;184:159–69.
Fukushima K, Inoue Y, Haga Y, Ota T, Honda K, Sato C, et al. Monoether-tagged biodegradable polycarbonate preventing platelet adhesion and demonstrating vascular cell adhesion: a promising material for resorbable vascular grafts and stents. Biomacromolecules. 2017;18:3834–43.
Lu N, Li P, Zhou L, Wang R, Chen H, Li X, et al. Synthesis, evaluation of phospholipid biomimetic polycarbonate for potential cardiovascular stents coating. React Funct Polym. 2021;163:104897.
Shen X, Su F, Dong J, Fan Z, Duan Y, Li S. In vitro biocompatibility evaluation of bioresorbable compolymers prepared from L-lactide, 1,3-trimethylene carbonate, and glycolide for cardiovascular applications. J Biomater Sci Polym Ed. 2015;26:497–514.
Kim SH, Tan JPK, Nederberg F, Fukushima K, Colson J, Yang C, et al. Hydrogen bonding-enhanced micelle assemblies for drug delivery. Biomaterials. 2010;31:8063–71.
Gao L, Dong B, Zhang J, Chen Y, Qiao H, Liu Z, et al. Functional biodegradable nitric oxide donor-containing polycarbonate-based micelles for reduction-triggered drug release and overcoming multidrug resistance. ACS Macro lett. 2019;8:1552–8.
Li Y, Wei J, Wei Y, Cheng L, Guo B, Meng F, et al. Apolipoprotein E pebtide-guided disulfide-cross-linked micelles for targeted delivery of sorafenib to hepatocellular carcinoma. Biomacromolecules. 2020;21:716–24.
Luo Y, Wu H, Zhou X, Wang J, Er S, Li Y, et al. J Am Chem Soc. 2023;145:20073–80.
Sun J, Birnbaum W, Anderski J, Picker MT, Mulac D, Langer K, et al. Biomacromolecules. 2018;19:4677–90.
Mohajeri S, Chen F, Prinse M, Phung T, Burke-Kleinman J, Maurice DH, et al. Liquid degradable poly(trimethylene-carbonate-co-5-hydroxy-trimethylene carbonate): an injectable drug delivery vehicle for acid-sensitive drugs. Mol Pharmaceutics. 2020;17:1363–76.
Barcan GA, Zhang X, Waymouth RM. Structurally dynamic hydrogels derived from 1,2-dithiolanes. J Am Chem Soc. 2015;137:5650–3.
Guillaume O, Geven MA, Sprecher CM, Stadelmann VA, Grijpma DW, Tang TT, et al. Surface-enrichment with hydroxyapatite nanoparticles in stereolithography-fabricated composite polymer scaffolds promotes bone repair. Acta Biomateriallia. 2017;54:386–98.
Brossier T, Volpi G, Vasques-Villegas J, Petitjean N, Guillaume O, Lapinte V, et al. Photoprintable gelatin-graft-poly(trimethylene carbonate) by stereolithography for tissue engineering applications. Biomacromolecules. 2021;22:3873–83.
Gielen AMC, Ankone M, Grijpma DW, Poot AA. Hybrid networks of hyaluronic acid and poly(trimethylene carbonate) for tissue regeneration. Biomacromolecules. 2023;24:4366–74.
Brissenden AJ, Amsden BG. In situ forming microporous biohybrid hydrogel for nucleus pulposus cell delivery. Acta Biomaterialia. 2023;170:169–84.
Ajiro H, Takahashi Y, Akashi M. Thermosensitive biodegradable homopolymer of trimethylene carbonate derivative at body temperature. Macromolecules. 2012;45:2668–74.
Chanthaset N, Takahashi Y, Haramiishi Y, Akashi M, Ajiro H. Control of thermoresponsivity of biocompatible poly(trimethylene carbonate) with direct introduction of oligo(ethylene glycol) under various circumstances. J Polym Sci Part A: Polym Chem. 2017;55:3466–74.
Nobuoka H, Miyake R, Choi J, Yoshida H, Chanthaset N, Ajiro H. Synthesis of ester-free type poly(trimethylene carbonate) derivatives bearing cycloalkyl side groups. Eur Polym J. 2021;160:110782.
Chanthset N, Maehara A, Ajiro H. Particles and film preparation of ester-free type poly(trimethylene carbonate) derivatives bearing aromatic groups initiated with hydrophilic initiators. Colloids Surf A. 2023;667:131413.
Tempelaar S, Mespouille L, Coulembier O, Dubois P, Dove AP. Synthesis and post-polymerization modifications of aliphatic poly(carbonate)s prepared by ring-opening polymerisation. Chem Soc Rev. 2013;42:1312–36.
Hassan M, Bhat GA, Darensbourg DJ. Post-polymerization functionalization of aliphatic polycarbonates using click chemistry. Polym Chem. 2024;15:1803–20.
Komforth P, Imschweiler J, Hesse M, Heck AG, Fuchs A, Hauck AV, et al. Toward intercellular delivery: aliphatic polycarbonates with pendant thiol-reactive thiosulfonates for reversible postpolymerization modification. Biomacromolecules. 2025;26:387–404.
Lanzi M, Kleij AW. Recent advances in the synthesis and polymerization of new CO2-based cyclic carbonates. Chin J Chem. 2024;42:430–43.
Nederberg F, Zhang Y, Tan JPK, Xu K, Wang H, Yang C, et al. Biodegradable nanostructures with selective lysis of microbial membranes. Nat Chem. 2011;3:409–14.
Ono RJ, Liu SQ, Venkataraman S, Chin W, Yang YY, Hedrick JL. Benzyl chloride-functionalized polycarbonates: a versatile platform for the synthesis of functional biodegradable polycarbonates. Macromolecules. 2014;47:7725–31.
Shi Y, Wang X, Graff RW, Phillip WA, Gao H. Synthesis of degradable molecular brushes via a combination of ring-opening polymerization and click chemistry. J Polym Sci Part A: Polym Chem. 2015;53:239–48.
Tempelaar S, Barker IA, Truong VX, Hall DJ, Mespouille L, Dubois P, et al. Organocatalytic synthesis and post-polymerization functionalization of propargyl-functional poly(carbonate)s. Polym Chem. 2013;4:174–83.
Tempelaar S, Mespouille L, Dubois P, Dove AP. Organocatalytic synthesis and post-polymerization functionalization of allyl-functional poly(carbonate)s. Macromolecules. 2011;44:2084–91.
Chen W, Yang H, Wang R, Cheng R, Meng F, Wei W, et al. Versatile synthesis of functional biodegradable polymers by combining ring-opening polymerization and post-polymerization modification via Michael-type addition reaction. Macromolecules. 2010;43:201–7.
Durand PL, Brège A, Chollet G, Grau E, Cramail H. Simple and efficient approach toward photosensitive biobased aliphatic polycarbonate materials. ACS Macro Lett. 2018;7:250–4.
Tan LY, Chanthaset N, Nanto S, Soba R, Nagasawa M, Ohno H, et al. Synthesis and preparation of cross-linked films with ester-free poly(trimethylene carbonate) bearing aromatic urea moiety. Macromolecules. 2021;54:5518–25.
Palenzuela M, Sarisuta K, Navarro M, Kumamoto N, Chanthaset N, Monat J, et al. 5-Methylene-1,3-dioxane-2-one: a first-choice comonomer for trimethylene carbonate. Macromolecules. 2023;56:678–89.
Totani M, Ando T, Terada K, Terashima T, Kim IY, Ohtsuki C, et al. Utilization of star-shaped polymer architecture in the creation of high-density polymer brush coatings for the prevention of platelet and bacteria adhesion. Biomater Sci. 2014;2:1172–85.
Palard I, Schappacher M, Belloncle B, Soum A, Guillaume SM. Unprecedented polymerization of trimethylene carbonate initiated by a samarium borohydride complex: mechanistic insights and copolymerization with ε-caprolactone. Chem A Eur J. 2007;13:1511–21.
Liu X, Liu X, Zheng H, Lu K, Chen D, Xiong C, et al. Improvement of hydrophilicity and formation of heparin/chitosan coating inhibits stone formation in ureteral stents. Colloids Surf A. 2024;694:134065.
Sivaraman B, Latour RA. The adherence of platelets to adsorbed albumin by receptor-mediated recognition of binding sites exposed by adsorption-induced unfolding. Biomaterials. 2010;31:1036–44.
Xu LC, Bauer JW, Siedlecki CA. Proteins, platelets, and blood coagulation at biomaterial interfaces. Colloids Surf B. 2014;124:49–68.
Yu F, Zhuo R. Synthesis, characterization, and degradation behaviors of end-group-functionalized poly(trimethylene carbonate)s. Polym J. 2003;35:671–6.
Acknowledgements
This work was supported financially by the Centre National de la Recherche Scientifique and the Université de Toulouse 3. This work was partly supported by the Suzuken Memorial Foundation and JSPS KAKENHI (JP24K01555). We are grateful for the fruitful discussions with Dr. Tsuyoshi Ando, Dr. Hiroaki Yoshida, Dr. Katsunori Tanaka, and Dr. Keita Okayama.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sarisuta, K., Martín-Vaca, B., Bourissou, D. et al. Thiol-ene derivatization of polycarbonates from 5-methylene-1,3-dioxane-2-one: an efficient and practical way to tune the surface biocompatibility of polycarbonate films. Polym J (2025). https://doi.org/10.1038/s41428-025-01068-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41428-025-01068-y