Abstract
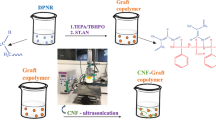
In this study, we investigated the graft copolymerization of triethylene glycol dimethacrylate (TEGDMA), a dimethacrylate monomer, onto natural rubber in its latex form. Graft copolymerization involves the reaction of TEGDMA with deproteinized natural rubber (DPNR) in the presence of a tetraethylenepentamine/tert-butyl hydroperoxide redox initiator. Two different initiator concentrations, 0.033 and 0.066 mol/kg rubber, as well as two monomer concentrations, 0.25 and 0.5 mol/kg rubber, were used. The resulting graft copolymers were characterized using X-ray diffraction (XRD), Fourier transform infrared (FTIR) spectroscopy and nuclear magnetic resonance (NMR) spectroscopy. Both FTIR and NMR analyses confirmed the successful grafting of TEGDMA onto DPNR, resulting in the formation of DPNR-graft-poly(TEGDMA). The properties of the graft copolymer were evaluated through various methods, including swelling tests, differential scanning calorimetry (DSC), tensile strength measurements, dynamic mechanical analysis (DMA), and thermogravimetric analysis (TGA). The gel content of DPNR increased dramatically after graft copolymerization, reaching 80–90%. Additionally, the glass transition temperature (Tg) shifted from −65.9 °C for DPNR to −67.7 °C for DPNR-graft-poly(TEGDMA) 0.033-0.5. Transmission electron microscopy (TEM) images revealed the formation of a poly(TEGDMA) layer surrounding the rubber molecules, creating a nanomatrix structure.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout












Similar content being viewed by others
References
Thuong NT, Oraphin Y, Nghia PT, Cornish K, Kawahara S. Effect of naturally crosslinking junctions on green strength of natural rubber. Polym Adv Technol. 2016;28:303–11.
Kangwansupamonkon W, Gilbert RG, Kiatkamjornwong S. Modification of Natural Rubber by Grafting with Hydrophilic Vinyl Monomers. Macromol Chem Phys. 2005;206:2450–60.
Promdum Y, Klinpituksa P, Ruamcharoen J. Graft copolymerization of natural rubber with 2-hydroxyethyl methacrylate for plywood adhesion improvement. Songklanakarin J Sci Technol. 2009;31:453–7.
Oliveira PC, Guimarães A, Cavaillé JY, Chazeau L, Gilbert RG, Santos AM. Poly(dimethylaminoethyl methacrylate) grafted natural rubber from seeded emulsion polymerization. Polymer. 2005;46:1105–11.
Yusof NH, Kosugi K, Song TK, Kawahara S. Preparation and characterization of poly(stearyl methacrylate) grafted natural rubber in latex stage. Polymer. 2016;88:43–51.
Angnanon S, Prasassarakich P, Hinchiranan N. Styrene/acrylonitrile graft natural rubber as compatibilizer in rubber blends. Polym-Plast Technol. 2011;50:1170–8.
Sari TI, Saputra AH, Bisco S, Maspanger DR, Cifriadi A. The effect of styrene monomer in the graft copolymerization of acrylonitrile onto deproteinzed natural rubber. Int J Technol. 2015;6:1164–73.
Anh NTN, Nam VT, Tue VM, Huy TT, Quynh NT, Hau TV, et al. Characterization of deproteinized natural rubber modified via graft copolymerization with styrene/acrylonitrile and reinforced with cellulose nanofibers. Polym J. 2025;57:1–12.
Nam VT, Quynh VT, Anh NTN, Nham DD, Hau TV, Tuyet TT, et al. Compatibility of deproteinized natural rubber-grafted methyl methacrylate and regenerated cellulose in their composite fabricated by co-precipitation. Polym Bull. 2024;81:8511–626.
Thuong NT, Huong DV, Ha CH, Hanh NTH, Nurul HY, Kawahara S. Preparation and properties of colloidal silica-filled natural rubber grafted with poly(methyl methacrylate). Polym Bull. 2021;79:6011–27.
Pfeifer CS, Shelton ZR, Braga RR, Windmoller D, Machado J, Stansbury JW. Characterization of dimethylacrylate polymeric networks: a study of the crosslinked structure formed by monomers used in dental composites. Eur Polym J. 2011;47:162–70.
Ceylan G, Emik S, Yalcinyuva T, Sunbuloğlu E, Bozdag E, Unalan F. The effects of cross-linking agents on the mechanical properties of poly (Methyl Methacrylate) Resin. Polymers. 2023;15:2387.
Maletin A, Ristic I, Veljovic T, Ramic B, Puskar T, Jeremic-Knezevic M, et al. Influence of dimethacrylate monomer on the polymerization efficacy of resin-based dental cements - FTIR analysis. Polymers. 2022;14:247.
Trofin MA, Racovita S, Vasiliu S, Bargan A, Bucatariu F, Vasiliu AL, et al. Synthesis of crosslinked microparticles based on glycidyl methacrylate and N-vinylimidazole. Macromol Chem Phys. 2023;224:2300253.
Chen L, Yu Q, Wang Y, Li H. BisGMA/TEGDMA dental composite containing high aspect-ratio hydroxyapatite nanofibers. Dent Mater. 2011;27:1187–95.
Wei F, Yu H, Zeng Z, Liu H, Wang Q, Wang J, et al. Preparation and structure characterization of hydroxylethylmethacrylate grafted natural rubber latex. Polimeros. 2014;24:283–90.
Pasek-Allen JL, Wiharm RK, Bischof JC, Pierre BC. NMR characterization of polyethylene glycol conjugates for nanoparticle functionalization. ACS Omega. 2023;8:4331–6.
Yamamoto Y, Suksawad P, Pukkate N, Horimai T, Wakisaka O, Kawahara S. Photoreactive nanomatrix structure formed by graft-copolymerization of 1,9-nonandiol dimethacryate onto natural rubber. J Polym Sci Part A Polym Chem. 2010;48:2418–24.
Kosugi K, Sutthangkul R, Chaikumpollert O, Yamamoto Y, Sakdapipanich J, Isono Y, et al. Preparation and characterization of natural rubber with soft nanomatrix structure. Colloid Polym Sci. 2012;290:1457–62.
Thuong NT, Ha CH, Nurul HY, Kawahara S. Graft copolymerization of methyl methacrylate and vinyltriethoxysilane binary monomer onto natural rubber. J Polym Res. 2021;28:246.
Thuong NT, Dung TA, Nurul HY, Kawahara S. Controlling the size of silica nanoparticles in filler nanomatrix structure of natural rubber. Polymer. 2020;195:12244.
Acknowledgements
This research is funded by Hanoi University of Science and Technology under grant number T2023-PC-100.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing of interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nghiem, T.T., Nguyen Thu, T., Yusof, N.H. et al. Graft copolymerization of triethylene glycol dimethacrylate onto natural rubber. Polym J 57, 1347–1357 (2025). https://doi.org/10.1038/s41428-025-01084-y
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41428-025-01084-y