Abstract
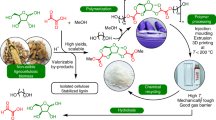
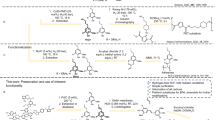
Owing to the increase in global concern, the replacement of fossil fuel-derived plastics with biomass polymers has attracted the interest of many researchers in both academia and industry. Levoglucosenone (LGO; 1), which is a biomass compound obtained from cellulose in wood or waste paper, has been used as a feedstock for biomass polymers. We recently discovered the stereoselective formation of tricomponent polymers, including LGO fragments, dicarboxylic dihydrazides and aromatic dithiol. In this report, the syntheses and properties of their analogs using aliphatic dithiol instead of aromatic dithiol are reported. The LGO monomer linked with 1,4-butanedithiol was prepared by the addition of K2CO3. Polycondensation of the monomer with several dicarboxylic dihydrazides proceeded to form the corresponding polyhydrazones. All the C-S and C = N bonds were formed stereoselectively. The obtained polymers showed negative optical rotations, which were complementary to those of aromatic analogs. When the polymerization time increased, DMF-insoluble polymers were obtained, which formed organogels with DMF. The degree of swelling varied between 170% and 469% depending on the carbon number of the dicarboxylic dihydrazide. The elongation of the aliphatic carbon chains was assumed to promote intermolecular entanglement, and the resulting polymers were assumed to behave as network polymers despite the linear chains.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Tang C, Ryu CY Sustainable Polymers from Biomass. Weinheim: Wiley-VCH; 2017.
Kudo S, Huang X, Asano S, Hayashi J-I. Catalytic strategies for levoglucosenone production by pyrolysis of cellulose and lignocellulosic biomass. Energy Fuels. 2021;35:9809–24.
Kudo S, Goto N, Sperry J, Norinaga K, Hayashi J-I. Production of levoglucosenone and dihydrolevoglucosenone by catalytic reforming of volatiles from cellulose pyrolysis using supported ionic liquid phase. ACS Sustainable Chem. Eng. 2017;5:1132–40.
Camp JE, Greatrex BW. Levoglucosenone: bio-based platform for drug discovery. Front. Chem. 2022;10:902239.
Kühlborn J, Groß J, Opatz T. Making natural products from renewable feedstocks: back to the roots? Nat. Prod. Rep. 2020;37:380–424.
Nishikawa T, Isobe M. Synthesis of Tetrodotoxin, a Classic but Still Fascinating Natural Product. Chem. Rec. 2013;13:286–302.
Liu X, Carr P, Gardiner MG, Banwell MG, Elbanna AH, Khalil ZG, et al. Levoglucosenone and Its Pseudoenantiomer iso-Levoglucosenone as Scaffolds for Drug Discovery and Development. ACS Omega. 2020;5:13926–39.
Müller C, Frau MAG, Ballinari D, Colombo S, Bitto A, Martegani E, et al. Design, Synthesis, and Biological Evaluation of Levoglucosenone-Derived Ras Activation Inhibitors. ChemMedChem. 2009;4:524–8.
Witczak ZJ, Sarnik J, Czubatka A, Forma E, Poplawski T. Thio-sugar motif of functional CARB-pharmacophore for antineoplastic activity. Part 2. Bioorg. Med. Chem. Lett. 2014;24:5606–11.
https://circa-group.com/products/lgo-derivatives/ (Accessed 21st April 2025).
Debsharma T, Yagci Y, Schlaad H. Cellulose-Derived Functional Polyacetal by Cationic Ring-Opening Polymerization of Levoglucosenyl Methyl Ether. Angew. Chem. Int. Ed. 2019;58:18492–5.
Wu L, Zhou Z, Sathe D, Zhou J, Dym S, Zhao Z, et al. Precision native polysaccharides from living polymerization of anhydrosugars. Nat. Chem. 2023;15:1276–84.
Mizukami Y, Kakehi Y, Li F, Yamamoto T, Tajima K, Isono T. Satoh. Chemically Recyclable Unnatural (1 → 6)-Polysaccharides from Cellulose-Derived Levoglucosenone and Dihydrolevoglucosenone. ACS Macro Lett. 2024;13:252–9.
Debsharma T, Behrendt FN, Laschewsky A, Schlaad H. Ring-Opening Metathesis Polymerization of Biomass-Derived Levoglucosenol. Angew. Chem. Int. Ed. 2019;58:6718–21.
Debsharma T, Schimdt B, Laschewsky A, Schlaad H. Ring-Opening Metathesis Polymerization of Unsaturated Carbohydrate Derivatives: Levoglucosenyl Alkyl Ethers. Macromolecules. 2021;54:2720–8.
Diot-Néant F, Mouterde LMM, Veith C, Couvreur J, Miller SA, Allais F. Sustainable one-pot synthesis and polycondensation of a levoglucosenone-derived cyclic acetal diol. ACS Sustainable Chem. Eng. 2022;10:10132–43.
Lombardo G, Warne CM, Damonte G, Todea A, Nagy L, Guebitz GM, et al. Sustainable synthesis of terpolyesters based on a levoglucosenone-derived cyclic acetal diol. ACS Sustainable Chem. Eng. 2025;13:4068–77.
Banwell MG, Liu X, Connal LA, Gardiner MG. Synthesis of functionally and stereochemically diverse polymers via ring-opening metathesis polymerization of derivatives of the biomass-derived platform molecule levoglucosenone produced at industrial scale. Macromolecules. 2020;53:5308–14.
Fadlallah S, Peru AAM, Flourat AL, Allais F. A straightforward access to functionalizable polymers through ring-opening metathesis polymerization of levoglucosenone-derived monomers. Eur. Polym. J. 2020;138:109980.
Wu L, Kim H, Choi T-L. Degradable alternating copolymers from living radical copolymerization of natural levoglucosenone and dienes. J. Am. Chem. Soc. 2025;147:11682–7.
Pollard B, Gardiner MG, Banwell MG, Connal LA. Polymers from cellulosic waste: direct polymerization of levoglucosenone using DBU as a catalyst. ChemSusChem. 2024;17:e202301165.
Tahara A, Yahiro S, Hokajo T, Kudo S, Yoshizaki Y, Konno T, et al. Stereoselective polycondensation of levoglucosenone leading to water-degradable biopolymers. Polym. Chem. 2025;16:800–8.
Prasad M, Timilsina, Stanfield MK, Smith JA, Thickett SC. Synthesis and characterization of thiol-ene networks derived from levoglucosenone. ChemPlusChem. 2024;89:e202400383.
Tahara A, CSD Commun. 2024;CCDC 2413468 https://doi.org/10.5517/ccdc.csd.cc2m0dtw.
Anuradha, Das A, Pal S, Jewrajka SK. Physical, electrochemical, and solvent permeation properties of amphiphilic conetwork membranes formed through interlinking of Poly(vinylidene fluoride)-Graft-Poly[(2-dimethylamino)ethyl Methacrylate] with Telechelic Poly(ethylene glycol) and small molecular weight cross-linkers. Langmuir. 2022;38:15340–52.
Stewart WE, Siddall TH. Nuclear magnetic resonance studies of amides. Chem. Rev. 1970;70:517–51.
Espsito CL, Kirilov P. Roullin V. Organogels, promising drug delivery systems: an update of state-of-the-art and recent applications. J. Control Release. 2018;271:1–20.
Bonnet J, Suissa G, Raynal MJ, Bouteikker L. Organogel formation rationalized by Hansen solubility parameters: dos and don’ts. Soft Matter. 2014;10:3154–60.
Matsumoto S, Yamaguchi S, Ueno S, Komatsu M, Ikeda M, Ishizuka K, et al. Photo gel–sol/sol–gel transition and its patterning of a supramolecular hydrogel as stimuli-responsive biomaterials. Chem. Eur. J. 2008;14:3977–86.
Stupp SI. Self-assembly and biomaterials. Nano Lett. 2010;10:4783–6.
Cintas P, Cravotto G. Molecular self-assembly and patterning induced by sound waves. The case of gelation. Chem. Soc. Rev. 2009;38:2684–97.
Maeda H. Anion-responsive supramolecular gels. Chem. Eur. J. 2008;14:11274–82.
Tang F, Ji C, Wu S, Yang Y, Huang F, Wei Q. Optimizing Poly(vinyl alcohol) organogel sensor performance at ultralow temperatures via synergistic modulation of forces and crystallization. ACS Appl. Polym. Mater. 2024;6:11350–9.
Salimi-Kenari H, Mollaie F, Dashtimoghadam E, Imani M, Nyström B. Effects of chain length of the cross-linking agent on rheological and swelling characteristics of dextran hydrogels. Carbohydr. Polym. 2018;181:141–9.
Matsushita S, Takano A, Takahashi Y, Matsushita Y. Dynamic viscoelasticity of a series of poly(4-n-alkylstyrene)s and their alkyl chain length dependence. Polymer. 2017;133:137–42.
Zheng D, Lin S, Ni J, Zhao X. Fracture and fatigue of entangled and unentangled polymer networks. Extreme Mechanics Lett. 2022;51:101608.
Liu Y, Xian W, He J, Li Y. Interplay between entanglement and crosslinking in determining mechanical behaviors of polymer networks. Int. J. Smart and Nano Mat. 2023;14:474–95.
Acknowledgements
This work was supported by a project (JPNP16002) subsidized by the New Energy and Industrial Technology Development Organization (NEDO) [A.T., T.H., and S.K.], JSPS KAKENHI Grant Numbers JP23K23357 [A.T.], JP22H05554 [A.T.], JP24H01304 [A.T.], JSPS LEADER Grant Number JPMXS0320200558 [A.T.] and JST SPRING Grant Number JPMJSP2114 [S.Y.]. The X-ray diffraction study for Compound 3 was conducted by Mr. Taisuke Matsumoto (Kyushu University).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
41428_2025_1088_MOESM1_ESM.pdf
Supplementary Information for Stereoselective Syntheses and Properties of Levoglucosenone-Containing Polymers Linked with 1,4-Butanedithiol and Their Application as Organogels
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yashiro, S., Ishikawa, H., Hokajo, T. et al. Stereoselective syntheses and properties of levoglucosenone-containing polymers linked with 1,4-butanedithiol and their application as organogels. Polym J 57, 1165–1175 (2025). https://doi.org/10.1038/s41428-025-01088-8
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41428-025-01088-8