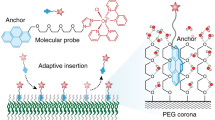
Abstract
The effects of the topology of cyclic polymers at surfaces and interfaces are described and discussed in this review. The topics include those at the air‒water interface, micellization, phase transition, and adsorption to nanoparticles. Surface tension studies revealed that the interfacial activity of cyclized polymers is greater than that of their linear counterparts because of an increase in the molecular density at the air‒water interface. Moreover, the micellization enthalpy and entropy (ΔHmic and ΔSmic) and lower critical micelle temperature (TCMT) were found for cyclic polymers, where the hydrophilic/hydrophobic ratio also significantly influenced. In addition, cyclic poly(ethylene glycol), c-PEG, can interact strongly with gold nanoparticles (AuNPs) and bovine serum albumin (BSA), whereas no such effects were found for linear PEG. The red color of an AuNPs dispersion vanished when BSA was added to complexes of AuNPs/c-PEG to form aggregates. In this context, silver nanoparticles (AgNPs) have not been proven to be capable of consistent PEGylation despite their usefulness in biological applications. c-PEG was found to physisorb onto AgNPs to effectively PEGylate and improve dispersion stability under physiological circumstances, long-term exposure to white light, and high temperature. Consequently, the generation of new functional materials and their applications can be facilitated by the effects of cyclization, which makes the use of a polymer topology feasible for the logical design of polymeric materials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Shao G, Li A, Liu Y, Yuan B, Zhang W. Branched polymers: synthesis and application. Macromolecules. 2023;57:830–46.
Jia Z, Monteiro MJ. Cyclic polymers: methods and strategies. J Polym Sci Part A Polym Chem. 2012;50:2085–97.
Laurent BA, Grayson SM. Synthetic approaches for the preparation of cyclic polymers. Chem. 2009;38:2202–13.
Kricheldorf HR, Schwarz G. Cyclic polymers by kinetically controlled step-growth polymerization. Macromol Rapid Commun. 2003;24:359–81.
Yamamoto T, Tezuka Y. Cyclic polymers revealing topology effects upon self-assemblies, dynamics and responses. Soft Matter. 2015;11:7458–68.
Watanabe T, Chimura S, Wang YB, Ono T, Isono T, Tajima K, et al. Cyclization of PEG and Pluronic surfactants and the effects of the topology on their interfacial activity. Langmuir. 2021;37:6974–84.
Watanabe T, Wang Y, Ono T, Chimura S, Isono T, Tajima K, et al. Topology and sequence-dependent micellization and phase separation of Pluronic L35, L64, 10R5, and 17R4: effects of cyclization and the chain ends. Polymers. 2022;14:1823.
Oziri OJ, Maeki M, Tokeshi M, Isono T, Tajima K, Satoh T, et al. Topology-dependent interaction of cyclic poly(ethylene glycol) complexed with gold nanoparticles against bovine serum albumin for a colorimetric change. Langmuir. 2022;38:5286–95.
Oziri OJ, Wang YB, Watanabe T, Uno S, Maeki M, Tokeshi M, et al. PEGylation of silver nanoparticles by physisorption of cyclic poly(ethylene glycol) for enhanced dispersion stability, antimicrobial activity, and cytotoxicity. Nanoscale Adv. 2022;4:532–45.
Schmolka IR. Artificial skin I. Preparation and properties of pluronic F-127 gels for treatment of burns. J Biomed Mater Res. 1972;6:571–82.
Prasad KN, Luong TT, Florence AT, Paris J, Vaution C, Seiller M, et al. Surface activity and association of ABA polyoxyethylene—polyoxypropylene block copolymers in aqueous solution. J Colloid Interface Sci. 1979;69:225–32.
Alexandridis P, Athanassiou V, Fukuda S, Hatton T. A. Surface activity of poly(ethylene oxide)-block-poly(propylene oxide)-block-poly(ethylene oxide) copolymers. Langmuir. 1994;10:2604–12.
Yu GE, Deng Y, Dalton S, Wang QG, Attwood D, Price C, et al. Micellisation and gelation of triblock copoly(oxyethylene/oxypropylene/oxyethylene), F127. J Chem Soc Faraday Trans. 1992;88:2537–44.
Yang Z, Pickard S, Deng NJ, Barlow RJ, Attwood D, Booth C. Effect of block structure on the micellization and gelation of aqueous solutions of copolymers of ethylene oxide and butylene oxide. Macromolecules. 1994;27:2371–9.
Varade D, Sharma R, Aswal VK, Goyal PS, Bahadur P. Effect of hydrotropes on the solution behavior of PEO/PPO/PEO block copolymer L62 in aqueous solutions. Eur Polym J. 2004;40:2457–64.
Yu GE, Garrett CA, Mai SM, Altinok H, Attwood D, Price C, et al. Effect of cyclization on the association behavior of block copolymers in aqueous solution. Comparison of Oxyethylene/Oxypropylene Block Copolymers Cyclo-P34E104 and E52P34E52. Langmuir. 1998;14:2278–85.
Hirose Y, Taira T, Sakai K, Sakai H, Endo A, Imura T. Structures and surface properties of “cyclic” polyoxyethylene alkyl ethers: unusual behavior of cyclic surfactants in water. Langmuir. 2016;32:8374–82.
Kim SH, Jo WH. A Monte Carlo simulation for the micellization of ABA- and BAB-type triblock copolymers in a selective solvent. Macromolecules. 2001;34:7210–18.
Kim SH, Jo WH. A Monte Carlo simulation for the micellization of ABA- and BAB-type triblock copolymers in a selective solvent. II. Effects of the block composition. J Chem Phys. 2002;117:8565–72.
Svard A, Neilands J, Palm E, Svensater G, Bengtsson T, Aili D. Protein-functionalized gold nanoparticles as refractometric nanoplasmonic sensors for the detection of proteolytic activity of porphyromonas gingivalis. ACS Appl Nano Mater. 2020;3:9822–30.
Hu SQ, Huang PJJ, Wang JX, Liu JW. Dissecting the effect of salt for more sensitive label-free colorimetric detection of DNA using gold nanoparticles. Anal Chem. 2020;92:13354–60.
Scari G, Porta F, Fascio U, Avvakumova S, Dal Santo V, De Simone M, et al. Gold nanoparticles capped by a GC-containing peptide functionalized with an RGD motif for integrin targeting. Bioconjugate Chem. 2012;23:340–9.
Wang GK, Papasani MR, Cheguru P, Hrdlicka PJ, Hill RA. Gold-peptide nanoconjugate cellular uptake is modulated by serum proteins. Nanomedicine. 2012;8:822–32.
Zhang G, Yang Z, Lu W, Zhang R, Huang Q, Tian M, et al. Influence of anchoring ligands and particle size on the colloidal stability and in vivo biodistribution of polyethylene glycol-coated gold nanoparticles in tumor-xenografted mice. Biomater. 2009;30:1928–36.
Nam S, Parikh DV, Condon BD, Zhao Q, Yoshioka-Tarver M. Importance of poly(ethylene glycol) conformation for the synthesis of silver nanoparticles in aqueous solution. J Nanopart Res. 2011;13:3755–64.
Wang Y, Quinsaat JEQ, Ono T, Maeki M, Tokeshi M, Isono T, et al. Enhanced dispersion stability of gold nanoparticles by the physisorption of cyclic poly(ethylene glycol). Nat Commun. 2020;11:6089.
Lacerda SHD, Park JJ, Meuse C, Pristinski D, Becker ML, Karim A, et al. Interaction of gold nanoparticles with common human blood proteins. ACS Nano. 2010;4:365–79.
Naveenraj S, Anandan S, Kathiravan A, Renganathan R, Ashokkumar M. The interaction of sonochemically synthesized gold nanoparticles with serum albumins. J Pharm Biomed Anal. 2010;53:804–10.
Pramanik S, Banerjee P, Sarkar A, Bhattacharya SC. Size-dependent interaction of gold nanoparticles with transport protein: a spectroscopic study. J Lumin. 2008;128:1969–74.
Kathiravan A, Paramaguru G, Renganathan R. Study on the binding of colloidal zinc oxide nanoparticles with bovine serum albumin. J Mol Struct. 2009;934:129–37.
Kathiravan A, Anandan S, Renganathan R. Interaction of colloidal TiO2 with human serum albumin: a fluorescence quenching study. Colloids Surf, A. 2009;333:91–5.
Brewer SH, Glomm WR, Johnson MC, Knag MK, Franzen S. Probing BSA binding to citrate-coated gold nanoparticles and surfaces. Langmuir. 2005;21:9303–7.
Xu L, Wang YY, Huang J, Chen CY, Wang ZX, Xie H. Silver nanoparticles: synthesis, medical applications and biosafety. Theranostics. 2020;10:8996–9031.
Singh A, Dar MY, Joshi B, Sharma B, Shrivastava S, Shukla S. Phytofabrication of silver nanoparticles: novel drug to overcome hepatocellular ailments. Toxicol Rep. 2018;5:333–42.
Saadmim F, Forhad T, Sikder A, Ghann W, Ali M. M, Sitther V, et al. Enhancing the performance of dye sensitized solar cells using silver nanoparticles modified photoanode. Molecules. 2020;25:4021.
Elhakim HKA, Azab SM, Fekry AM. A novel simple biosensor containing silver nanoparticles/propolis (bee glue) for microRNA let-7a determination. Mater Sci Eng, C. 2018;92:489–95.
Huy TQ, Huyen PTM, Le AT, Tonezzer M. Recent advances of silver nanoparticles in cancer diagnosis and treatment: anti-Cancer. Agents Med Chem. 2020;20:1276–87.
Pandian N, Chidambaram S. Antimicrobial, cytotoxicty and anti cancer activity of silver nanoparticles from glycyrrhiza glabra. Int J Pharm Sci Res. 2017;8:1633–41.
Bagherzade G, Tavakoli MM, Namaei MH. Green synthesis of silver nanoparticles using aqueous extract of saffron (Crocus sativus L.) wastages and its antibacterial activity against six bacteria: Asian Pac. J Trop Biomed. 2017;7:227–33.
Manson J, Kumar D, Meenan BJ, Dixon D. Polyethylene glycol functionalized gold nanoparticles: the influence of capping density on stability in various media. Gold Bull. 2011;44:99–105.
Marchioni M, Battocchio C, Joly Y, Gateau C, Nappini S, Pis I, et al. Thiolate-capped silver nanoparticles: discerning direct grafting from sulfidation at the metal-ligand interface by interrogating the sulfur atom. J Phys Chem C. 2020;124:13467–78.
Liu L, Burnyeat CA, Lepsenyi RS, Nwabuko IO, Kelly TL. Mechanism of shape evolution in Ag nanoprisms stabilized by thiol-terminated poly(ethylene glycol): an in situ kinetic study. Chem Mater. 2013;25:4206–14.
Lee JS, Kim H, Algar WR. Thiol-ligand-catalyzed quenching and etching in mixtures of colloidal quantum dots and silver nanoparticles. J Phys Chem C. 2017;121:28566–75.
Cheng YW, Yin LY, Lin SH, Wiesner M, Bernhardt E, Liu J. Toxicity reduction of polymer-stabilized silver nanoparticles by sunlight. J Phys Chem C. 2011;115:4425–32.
Acknowledgements
This work was supported by Grant-in-Aid for Challenging Research (Pioneering) (22K18334, TY), Grant-in-Aid for Scientific Research (B) (21H01991, TY), the Asahi Glass Foundation (TY), and the Iketani Science and Technology Foundation (TY).
Author information
Authors and Affiliations
Contributions
TY and JT wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yamamoto, T., Tazuke, J. Unique properties of cyclic polymers at interfaces and their applications to nanomaterials. Polym J (2025). https://doi.org/10.1038/s41428-025-01095-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41428-025-01095-9