Abstract
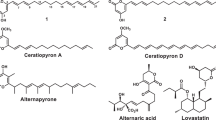
Cordybislactone (3), a new stereoisomer of the 14-membered bislactone clonostachydiol, together with its open ring analog (4), was isolated from the hopper pathogenic fungus Cordyceps sp. BCC 49294. The relative and absolute configurations of 3 were determined by chemical derivatizations, including the modified Mosher’s method. The stereochemistry of clonostachydiol was determined using the natural compound isolated from Xylaria sp. BCC 4297. The result revealed that the absolute configuration of clonostachydiol, previously determined by synthesis, should be revised to its enantiomer.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Grabley S, Hammann P, Thiericke R, Wink J, Philipps S, Zeeck A. Secondary metabolites from chemical screening. 21 Clonnostachydiol, a novel anthelmintic macrodiolide from the fungus Clonostachys cylindrospora (strain FH-A 6607). J Antibiot, (1993 46, 343–5.
Zeek A, Philipps S, Kind, R, Grabley S, Hammann P, Thiericke R, Wink J, Düwel D, Schmid K. Production of clonostachydiol and helmidiol, novel macrolides with anthelmintic activity. DE 4225284A1 (1992).
Rama Rao AV, Murthy VS, Sharma, GVM. The first synthesis and determination of absolute stereochemistry of clonostachydiol – Part II. Tetrahedron Lett, 1995;36:143–6.
Rama Rao AV, Murthy VS, Sharma GVM. Studies directed towards the synthesis of clonostachydiol – Part I. Tetrahedron Lett, 1995;36:139–42.
Yadav JS, Swamy T, Subba Reddy BV. A stereoselective approach for the total synthesis of clonostachydiol. Synlett, (No. 18), 2008:2773–6.
Ramulu U, Ramesh D, Rajaram S, Reddy SP, Venkatesham K, Venkateswarlu Y. Stereoselective total synthesis of clonostachydiol. Tetrahedron Assymmetry, 2012;23:117–23.
Lang G, Mitova MI, Ellis G, van der Sar S, Phipps RK, Blunt JW, Cummings NJ, Cole ALJ, Munro MHG. Bioactivity profiling HPLC/microtiter-plate analysis: application to a New Zealand marine alga-derived fungus, Gliocladium sp. J Nat Prod, 2006;69:621–4.
Abate D, Abraham, W.-R, Meyer, H. Cytochalasins and phytotoxins from the fungus Xylaria obovata. Phytochemistry, 1997;44:1443–8.
Isaka M, Yangchum A, Auncharoen P, Srichomthong K, Srikitikulchai P. Ring B aromatic norpimarane glucoside from a Xylaria sp. J Nat Prod, 2011;74:300–2.
Han J, Su Y, Jiang T, Xu Y, Huo X, She X, Pan X. Asymmetric total synthesis and revision of the absolute configuration of 4-keto-clonostachydiol. J Org Chem, 2009;74:3930–2.
Ohtani I, Kusumi T, Kashman Y, Kakisawa H. High-field FT NMR application of Mosher’s method. The absolute configurations of marine terpenoids. J Am Chem Soc, 1991;113:4092–6.
O’Brien J, Wilson I, Orton T, Pognan F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur J Biochem, 2000;267:5421–6.
Changsen C, Franzblau SG, Palittapongarnpim P. Improved green fluorescent protein reporter gene-based microplate screening for antituberculosis compounds by utilizing an acetamidase promoter. Antimicrob Agents Chemother, 2003;47:3682–7.
Acknowledgements
Financial support from the Thailand Research Fund (Grant No. DBG5980002) and JSPS KAKENHI (Grant Numbers 16H01127, 16H00999, and a Grant-in-aid for Scientific Research (A) 26253001) is gratefully acknowledged. Ojima thanks JSPS KAKENHI, Grant-in-aid for Scientific Research Priority Area: Middle Molecular Strategy. We are grateful to Mr Prasert Srikitikulchai for identification of the fungus Xylaria sp. BCC 4297.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Ojima, Ki., Yangchum, A., Laksanacharoen, P. et al. Cordybislactone, a stereoisomer of the 14-membered bislactone clonostachydiol, from the hopper pathogenic fungus Cordyceps sp. BCC 49294: revision of the absolute configuration of clonostachydiol. J Antibiot 71, 351–358 (2018). https://doi.org/10.1038/s41429-017-0008-9
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41429-017-0008-9
This article is cited by
-
Recent progress in biodiversity research on the Xylariales and their secondary metabolism
The Journal of Antibiotics (2021)