Abstract
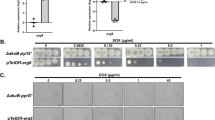
In a previous study on discovering new antimicrobial agents from microbial sources, nine bafilomycins were isolated from the fermentation broth of Streptomyces albolongus. Among them, bafilomycin C1 (Baf C1) showed strong antifungal activity against Candida albicans, with MIC value of 1.56 μg/mL. The aim of this study was to evaluate the action mechanism of Baf C1 against C. albicans. Quantitative PCR analysis revealed that ergosterol biosynthesis-related genes of C. albicans ACS1, HMG1, IDI1, ERG1, ERG2, ERG6, ERG7, ERG8, ERG9, ERG12, ERG13, ERG20, ERG24, ERG251, ERG252, ERG26, ERG27, and ERG28 were all down-regulated (Log2fold change < −1) after Baf C1(4 μg/mL) exposure. Moreover, the expression of MET6 gene, encoded methionine synthase, was also down-regulated (2.7-fold). It is corresponding with the quantitative PCR result, the content of ergosterol has dropped about 41% compared with the control. Transmission electron microscope examination also revealed that the Baf C1 strongly destroyed the cell membrane of C. albicans. In addition, the content of farnesol was significantly increased, about 2.1-fold compared with the control. The results indicated Baf C1 caused aberrations in sterol biosynthesis, leaded to the lack of ergosterol of the fungal membrane.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Zhang JD, et al. Antifungal activities and action mechanisms of compounds from Tribulus terrestris L. J Ethnopharmacol. 2006;103:76–84.
Wu XZ, Chang WQ, Cheng AX, Sun LM, Lou HX. Plagiochin E, an antifungal active macrocyclic bis(bibenzyl), induced apoptosis in Candida albicans through a metacaspase-dependent apoptotic pathway. Biochim Biophys Acta. 2010;1800:439–47.
Wu XZ, Cheng AX, Sun LM, Sun SJ, Lou HX. Plagiochin E, an antifungal bis(bibenzyl), exerts its antifungal activity through mitochondrial dysfunction-induced reactive oxygen species accumulation in Candida albicans. Biochim Biophys Acta. 2009;1790:770–7.
Jones T, et al. The diploid genome sequence of Candida albicans. Proc Natl Acad Sci USA. 2004;101:7329–34.
Fridkin SK, Jarvis WR. Epidemiology of nosocomial fungal infections. Clin Microbiol Rev. 1996;9:499–511.
Henry KW, Nickels JT, Edlind TD. Upregulation of ERG genes in Candida Species by azoles and other sterol biosynthesis inhibitors. Antimicrob Agents Chemother. 2000;44:2693–700.
Lyman CA, Walsh TJ. Systemically administered antifungal agents: a review of their clinical pharmacology and therapeutic applications. Drugs. 1992;44:9–35.
Sobel JD, et al. Fluconazole susceptibility of vaginal isolates obtained from women with complicated Candida vaginitis: clinical implications. Antimicrob Agents Chemother. 2003;47:34–8.
Ding N, et al. Bafilomycins and odoriferous sesquiterpenoids from Streptomyces albolongus isolated from Elephas maximus feces. J Nat Prod. 2016;79:799–805.
Werner G, Hagenmaier H, Drautz H, Baumgartner A, Zähner H. Metabolic products of microorganisms. 224. Bafilomycins, a new group of macrolide antibiotics. Production, isolation, chemical structure and biological activity. J Antibiot. 1984;37:110–7.
Masand M, Jose PA, Menghani E, Jebakumar SRD. Continuing hunt for endophytic actinomycetes as a source of novel biologically active metabolites. World J Microbiol Biotechnol. 2015;31:1863–75.
Jedlickova L, Gadas D, Havlova P, Havel J. Determination of ergosterol levels in barley and malt varieties in the Czech Republic via HPLC. J Agric Food Chem. 2008;56:4092–5.
Arthington-Skaggs BA, Jradi H, Desai T, Morrison CJ. Quantitation of ergosterol content: novel method for determination of fluconazole susceptibility of Candida albicans. J Clin Microbiol. 1999;37:3332–7.
Anderson P, Davidson CM, Littlejohn D, Allan MU. Extraction of ergosterol from peaty soils and determination by high performance liquid chromatography. Talanta. 1994;41:711–20.
Newell SY, Arsuffi TL, Fallon RD. Fundamental procedures for determining ergosterol content of decaying plant material by liquid chromatography. Appl Environ Microbiol. 1988;54:1876–9.
Bachhawat AK, Yadav AK. Metabolic pathways as drug targets: targeting the sulphur assimilatory pathways of yeast and fungi for novel drug discovery. In: Ahmad I, Owais M, Shahid M, Aqil F, editors. Combating fungal infections: problems and remedy. New York: Springer; 2010. p. 327–46.
te Welscher YM, et al. Natamycin blocks fungal growth by binding specifically to ergosterol without permeabilizing the membrane. J Biol Chem. 2008;283:6393–401.
Souza AC, et al. Candida parapsilosis resistance to fluconazole: molecular mechanisms and in vivo impact in infected Galleria mellonella larvae. Antimicrob Agents Chemother. 2015;59:6581–7.
Berkow EL, et al. Multidrug transporters and alterations in sterol biosynthesis contribute to azole antifungal resistance in Candida parapsilosis. Antimicrob Agents Chemother. 2015;59:5942–50.
Bammert GF, Fostel JM. Genome-wide expression patterns in Saccharomyces cerevisiae: comparison of drug treatments and genetic alterations affecting biosynthesis of ergosterol. Antimicrob Agents Chemother. 2000;44:1255–65.
Parveen M, et al. Response of Saccharomyces cerevisiae to a monoterpene: evaluation of antifungal potential by DNA microarray analysis. J Antimicrob Chemother. 2004;54:46–55.
Wibawa T, Praseno, Aman AT. Virulence of Candida albicans isolated from HIV infected and non infected individuals. Springerplus. 2015;4:408.
Kovács R, et al. Effect of caspofungin and micafungin in combination with farnesol against Candida parapsilosisbiofilms. Int J Antimicrob Agents. 2016;47:304–10.
Munayyer HK, et al. Posaconazole is a potent inhibitor of sterol 14alpha-demethylation in yeasts and molds. Antimicrob Agents Chemother. 2004;48:3690–6.
Hornby JM, et al. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl Environ Microbiol. 2001;67:2982–92.
Ding M, Lu J, Zhao C, Zhang S, Zhao Y. Determination of 25-OCH3-PPD and the related substances by UPLC-MS/MS and their cytotoxic activity. J Chromatogr B. 2016;1022:274–80.
Georgopapadakou NH, Walsh TJ. Antifungal agents: chemotherapeutic targets and immunologic strategies. Antimicrob Agents Chemother. 1996;40:279–91.
Brajtburg J, Powderly WG, Kobayashi GS, Medoff G. Amphotericin B: current understanding of the mechanism of action. Antimicrob Agents Chemother. 1990;34:183–8.
Zhang L, et al. Response of gene expression in Saccharomyces cerevisiae to amphotericin B and nystatin measured by microarrays. J Antimicrob Chemother. 2002;49:905–15.
Alcazar-Fuoli L, et al. Ergosterol biosynthesis pathway in Aspergillus fumigatus. Steroids. 2008;73:339–47.
Starai VJ, Escalante-Semerena JC. Acetyl-coenzyme A synthetase (AMP forming). Cell Mol Life Sci. 2004;61:2020–30.
Corey EJ, Matsuda SP, Bartel B. Molecular cloning, characterization, and overexpression of ERG7, the Saccharomyces cerevisiae gene encoding lanosterol synthase. Proc Natl Acad Sci USA. 1994;91:2211–5.
Pascon RC, Ganous TM, Kingsbury JM, Cox GM, McCusker JH. Cryptococcus neoformans methionine synthase: expression analysis and requirement for virulence. Microbiology. 2004;150:3013–23.
Hwang JY, Kim HS, Kim SH, Oh HR, Nam DH. Organization and characterization of a biosynthetic gene cluster for bafilomycin from Streptomyces griseus DSM 2608. AMB Express. 2013;3:24.
Zhang YQ, et al. Requirement for ergosterol in V-ATPase function underlies antifungal activity of azole drugs. PLoS Pathog. 2010;6:e1000939. https://doi.org/10.1371/journal.ppat.1000939.
Alonso-Monge R, et al. Role of the mitogen-activated protein kinase Hog1p in morphogenesis and virulence of Candida albicans. J Bacteriol. 1999;181:3058–68.
Cao YY, et al. cDNA microarray analysis of differential gene expression in Candida albicans biofilm exposed to farnesol. Antimicrob Agents Chemother. 2005;49:584–9.
Chang WQ, et al. Retigeric acid B exerts antifungal effect through enhanced reactive oxygen species and decreased cAMP. Biochim Biophys Acta. 2011;1810:569–76.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant numbers 81573327, 81500934), and the Basic Scientific Research Fund of Northeastern University, China (grant number N142002001).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Su, H., Han, L., Ding, N. et al. Bafilomycin C1 exert antifungal effect through disturbing sterol biosynthesis in Candida albicans. J Antibiot 71, 467–476 (2018). https://doi.org/10.1038/s41429-017-0009-8
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41429-017-0009-8
This article is cited by
-
Exploring the Potential of Farnesol as a Novel Antifungal Drug and Related Challenges
Current Infectious Disease Reports (2024)
-
Molecular targets for antifungals in amino acid and protein biosynthetic pathways
Amino Acids (2021)
-
A semisynthetic borrelidin analogue BN-3b exerts potent antifungal activity against Candida albicans through ROS-mediated oxidative damage
Scientific Reports (2020)
-
Global proteomic analysis deciphers the mechanism of action of plant derived oleic acid against Candida albicans virulence and biofilm formation
Scientific Reports (2020)
-
Bafilomycin C1 induces G0/G1 cell-cycle arrest and mitochondrial-mediated apoptosis in human hepatocellular cancer SMMC7721 cells
The Journal of Antibiotics (2018)