Abstract
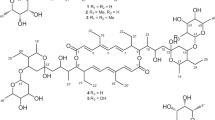
Two new compounds, designated as hamuramicins A (1) and B (2), were isolated from the cultured broth of an endophytic actinomycete Allostreptomyces sp. K12-0794 by silica gel column chromatography and HPLC. The structures of 1 and 2 were elucidated as 22-membered macrolide containing triene and trienone with an alkyl side chain by spectroscopic analyses including NMR experiments. Both compounds showed growth inhibition activity against Kocuria rhizophia and Xanthomonas oryzae pv. oryzae as well as human cell line toxicity.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Tiwari K, Gupta RK. Rare actinomycetes: a potential storehouse for novel antibiotics. Crit Rev Biotechnol. 2012;32:108–32.
Ōmura S, et al. A new alkaloid AM-2282 OF Streptomyces origin. Taxonomy, fermentation, isolation and preliminary characterization. J Antibiot. 1977;30:275–82.
Ōmura S, et al. Clostomicins, new antibiotics produced by Micromonospora echinospora subsp. Armeniaca subsp. nov. I. Production, isolation, and physico-chemical and biological properties. J Antibiot. 1986;39:1407–1412.
Ōmura S, Otoguro K, Nishikiori T, Ōiwa R, Iwai Y. Setamycin, a new antibiotic. J Antibiot. 1981;34:1253–6.
Nakashima T, et al. Mangromicins A and B: structure and antitrypanosomal activity of two new cyclopentadecane compounds from Lechevalieria aerocolonigenes K10-0216. J Antibiot. 2014;67:253–60.
Nakashima T, Kamiya Y, Iwatsuki M, Takahashi Y, Ōmura S. Mangromicins, six new anti-oxidative agents isolated from a culture broth of the actinomycete, Lechevalieria aerocolonigenes K10-0216. J Antibiot. 2014;67:533–9.
Nakashima T, et al. Mangromicin C, a new analog of mangromicin. J Antibiot. 2015;68:220–2.
Kimura T, et al. Anti-trypanosolam compound, sagamilactam, a new polyene macrocyclic lactam from Actinomadura sp. K13-0306. J Antibiot. 2016;69:818–24.
Nakashima T, Takahashi Y, Ōmura S. Search for new compounds from Kitasato microbial library by physicochemical screening. Biochem Pharmacol. 2016;134:42–55.
Matsumoto A, Takahashi Y. Endophytic actinomycetes: promising source of novel bioactive compounds. J Antibiot. 2017;70:514–9.
Takahashi Y. Continuing fascination of exploration in natural substances from microorganisms. Biosci Biotech Biochem. 2017;81:6–12.
Qin S, et al. Biodiversity, bioactive natural products and biotechnological potential of plant-associated endophytic actinobacteria. Appl Microbiol Biotechnol. 2011;89:457–73.
Inahashi Y, et al. Spoxazomicins A-C, novel antitrypanosomal alkaloids produced by an endophytic actinomycete. Streptosporangium oxazolinicum K07-0460T. J Antibiot. 2011;64:303–7.
Nakashima T, et al. Trehangelins A, B and C, novel photo-oxidative hemolysis inhibitors produced by an endophytic actinomycete, Polymorphospora rubra K07-0510. J Antibiot. 2013;66:311–7.
Inahashi Y, et al. Actinoallolides A-E, new anti-trypanosomal macrolides, produced by an endophytic actinomycete. Actinoallomurus fulvus MK10-036. Org Lett. 2015;17:864–7.
Huang MJ, et al. Allostreptomyces psammosilenae gen. nov., sp. nov., an endophytic actinobacterium isolated from the roots of Psammosiline tunicoides and emended description of the family Streptomyces [Waksman and Henrici (1943)AL] emend. Rainey et al. 1977, emend. Kim et al. 2003, emend Zhi et al. 2009. Int J Syst Evol Microbiol. 2017;67:288–93.
Smith JR, et al. Structure revision of the antibiotic pulvomycin. J Am Chem Soc. 1985;107:2849–57.
Parmeggiani A, et al. Structure basis of the action of pulvomycin and GE2270 A on elongation factor Tu. Biochemistry. 2006;45:6846–57.
Clinical and Laboratory Standards Institute, 2005. Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria; Proposed Guideline M45-P, CLSI, Wayne, USA
Landini P, Bandera M, Soffientini A, Goldstein BP. Sensitivity of elongation factor Tu (EF-Tu) from different bacterial species to the antibiotics efrotomycin, pulvomycin and MDL 62879. J Gen Microbiol. 1933;139:769–74.
Acknowledgements
This study was supported by funds from the Institute for Fermentation Osaka (IFO), Japan and JSPS KAKENHI (Grant Number 16H07167). We are grateful to Dr. Kenichiro Nagai and Ms. Noriko Sato, School of Pharmacy, Kitasato University for measurements of mass and NMR spectra.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Suga, T., Kimura, T., Inahashi, Y. et al. Hamuramicins A and B, 22-membered macrolides, produced by an endophytic actinomycete Allostreptomyces sp. K12-0794. J Antibiot 71, 619–625 (2018). https://doi.org/10.1038/s41429-018-0055-x
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41429-018-0055-x
This article is cited by
-
A new polycyclic tetramate macrolactam from Allostreptomyces RD068384: stereochemistry and antifungal potential
The Journal of Antibiotics (2024)
-
Emblestatin: a new peptide antibiotic from Embleya scabrispora K20-0267
The Journal of Antibiotics (2023)
-
Novel WYL domain-containing transcriptional activator acts in response to genotoxic stress in rapidly growing mycobacteria
Communications Biology (2023)
-
The Bacterial and Fungal Microbiota of the Mexican Rubiaceae Family Medicinal Plant Bouvardia ternifolia
Microbial Ecology (2022)
-
A review of approaches to control bacterial leaf blight in rice
World Journal of Microbiology and Biotechnology (2022)