Abstract
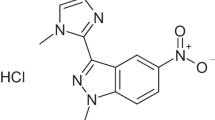
With antibiotics resistance developing rapidly, new antibacterial agents are needed to be discovered. We readily synthesized 11 indolin-2-one compounds and found a hybrid of indolin-2-one and nitroimidazole 3-((1-methyl-5-nitro-1H-imidazol-2-yl)methylene)indolin-2-one to be effective on Staphylococcus aureus strains. Six derivatives of this compound were further designed and synthesized in order to enhance its efficacy. After a second turn of structural refinement, a novel hybrid of indolin-2-one and nitroimidazole 3-((1-methyl-5-nitro-1H-imidazol-2-yl)methylene)-5-nitroindolin-2-one with a nitro group on C-5 position of indolin-2-one was shown to exhibit remarkable antibacterial activities with a low MIC value against MRSA ATCC 33591. Besides, this molecule demonstrated its potency on Gram-negative bacteria and VRE strain. The time-killing curve experiment showed its good bactericidal activity. Low hemolytic rate suggested its promising safety profile.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Boucher HW, et al. Bad bugs, no drugs: no ESKAPE! an update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1–12.
Willyard C. The drug-resistant bacteria that pose the greatest health threats. Nature. 2017;543:15.
Kaur M, Singh M, Chadha N, Silakari O. Oxindole: a chemical prism carrying plethora of therapeutic benefits. Eur J Med Chem. 2016;123:858–94.
Rajesh Kumar M,Alagumuthu M,Violet Dhayabaran V, N-substituted hydroxynaphthalene imino-oxindole derivatives as new class of PI3-kinase inhibitor and breast cancer drug: molecular validation and structure-activity relationship studies. Chem Biol Drug Des. 2018;91:277–84.
Ho HK, et al. Benzylidene-indolinones are effective as multi-targeted kinase inhibitor therapeutics against hepatocellular carcinoma. Mol Oncol. 2014;8:1266–77.
Ji C, et al. Design, synthesis and biological evaluation of novel antitumor spirotetrahydrothiopyran–oxindole derivatives as potent p53-MDM2 inhibitors. Bioorg Med Chem. 2017;25:5268–77.
Romagnoli R, et al. Design, synthesis and biological evaluation of 3-substituted-2-oxindole hybrid derivatives as novel anticancer agents. Eur J Med Chem. 2017;134:258–70.
Ashraf Ali M, et al. AChE inhibitor: a regio- and stereo-selective 1,3-dipolar cycloaddition for the synthesis of novel substituted 5,6-dimethoxy spiro[5.3′]-oxindole-spiro-[6.3″]-2,3-dihydro-1H-inden-1″-one-7-(substituted aryl)-tetrahydro-1H-pyrrolo[1,2-c][1,3]thiazole. Bioorg Med Chem Lett. 2012;22:508–11.
Akrami H, et al. Indolinone-based acetylcholinesterase inhibitors: synthesis, biological activity and molecular modeling. Eur J Med Chem. 2014;84:375–81.
Sun Y, et al. One-step synthesis of chiral oxindole-type analogues with potent anti-inflammatory and analgesic activities. Sci Rep. 2015;5:13699.
Chen G, et al. Synthesis and biological evaluation of novel indole-2-one and 7-aza-2-oxindole derivatives as anti-inflammatory agents. Drug Des Devel Ther. 2014; 8: 1869–92.
MIDOH N, et al. Antioxidative activities of oxindole-3-acetic acid derivatives from supersweet corn powder. Biosci Biotechnol Biochem. 2010;74:1794–801.
Ahmad I, et al. Xanthine oxidase/tyrosinase inhibiting, antioxidant, and antifungal oxindole alkaloids from Isatis costata. Pharm Biol. 2010;48:716–21.
Furuta K, Mizuno Y, Maeda M, Koyama H, Hirata Y. Synthesis of 3-arylmethyl-2-oxindole derivatives and their effects on neuronal cell death. Chem Pharm Bull. 2017;65:1093–7.
Furuta K, et al. Synthesis of 3-[4-(dimethylamino)phenyl]alkyl-2-oxindole derivatives and their effects on neuronal cell death. Bioorg Med Chem Lett. 2017;27:4457–61.
Gholamzadeh P, Mohammadi Ziarani G, Badiei A, Abolhassani Soorki A, Lashgari N. Efficient green synthesis of isoindigo derivatives using sulfonic-acid-functionalized nanoporous silica (SBA-Pr-SO3H) catalyst and study of their antimicrobial properties. Res Chem Intermed. 2013;39:3925–36.
Hosseinzadeh N, et al. 5-Nitro-heteroarylidene analogs of 2-thiazolylimino-4-thiazolidinones as a novel series of antibacterial agents. Med Chem Res. 2013;22:2293–302.
Batista A, et al. Antimicrobial effects of violacein against planktonic cells and biofilms of Staphylococcus aureus. Molecules. 2017;22:1534.
Rindhe SS, Karale BK, Gupta RC, Rode MA. Synthesis, antimicrobial and antioxidant activity of some oxindoles. Indian J Pharm Sci. 2011;73:292–6.
Li M-C, et al. Four new minor brominated indole related alkaloids with antibacterial activities from Laurencia similis. Bioorg Med Chem Lett. 2016;26:3590–3.
Majik MS, Rodrigues C, Mascarenhas S, D’Souza L. Design and synthesis of marine natural product-based 1H-indole-2,3-dione scaffold as a new antifouling/antibacterial agent against fouling bacteria. Bioorg Chem. 2014;54:89–95.
Acharya AP, et al. Green method for synthesis of 3-[2-(substituted-phenyl)-2-oxo ethylidene]-1,3-dihydro-indol-2-one and their in vitro antimicrobial activity. Res Chem Intermed. 2015;41:2953–9.
Winkelmann E,Raether W,Gebert U,Sinharay A, Chemotherapeutically active nitro compounds. 4. 5-Nitroimidazoles (part I). Arzneimittelforschung. 1977;27:2251–63.
Ang CW, Jarrad AM, Cooper MA, Blaskovich MAT. Nitroimidazoles: molecular fireworks that combat a broad spectrum of infectious diseases. J Med Chem. 2017;60:7636–57.
Acknowledgments
This work was financially supported by grants from the National Natural Science Foundation of China (Grant NO. 81602956 and 81473253), National Major Program of China during the 13th Five-Year Plan Period (Grant NO. 2018ZX09721001-001-001), and China Postdoctoral Science Foundation (Grant NO. 2016M590895). Thank Sichuan Provincial People’s Hospital for the supply of VRE B148 strains.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Zhou, Y., Ju, Y., Yang, Y. et al. Discovery of hybrids of indolin-2-one and nitroimidazole as potent inhibitors against drug-resistant bacteria. J Antibiot 71, 887–897 (2018). https://doi.org/10.1038/s41429-018-0076-5
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41429-018-0076-5
This article is cited by
-
Indole derivatives as antibacterials: overcoming MRSA resistance through SAR insights and AI-driven design
Proceedings of the Indian National Science Academy (2026)
-
Design and synthesis of novel spirooxindole–indenoquinoxaline derivatives as novel tryptophanyl-tRNA synthetase inhibitors
Molecular Diversity (2020)
-
Synthesis and antibacterial activity of 3-substituted 1-(2-methyl-5-nitrophenyl)-5-oxopyrrolidine derivatives
Research on Chemical Intermediates (2019)