Abstract
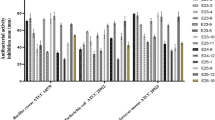
Novel benzoxaborole derivatives of azithromycin in which benzoxaborole residue is attached to the 4″-hydroxy-group of azithromycin have been synthesized. Antibacterial activity of synthesized derivatives in comparison with azithromycin was tested on a panel of Gram-positive and Gram-negative bacterial strains. All the investigated compounds demonstrated broad spectrum of antibacterial activity being in general more active against Gram-positive strains. New benzoxaborole derivatives of azithromycin demonstrated high activity against Streptococcus pyogenes ATCC 19615 and Propionibacterium acnes ATCC 6919 strains. Some of the new compounds were more active than azithromycin against Streptococcus pneumoniae ATCC 49619 strain or Enterococcus faecium strains. Using a reporter construct created on the basis of the transcription attenuator region of the Escherichia coli tryptophan operon pRFPCER-TrpL2A it has been demonstrated that the mechanism of action of azithromycin analogs is blocking nascent peptide in ribosome tunnel.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Tevyashova AN, Olsufyeva EN, Preobrazhenskaya MN. Design of dual action antibiotics as an approach to search for new promising drugs. Russ Chem Rev. 2015;84:61–97.
Pokrovskaya V, Baasov T. Dual-acting hybrid antibiotics: a promising strategy to combat bacterial resistance. Expert Opin Drug Discov. 2010;5:883–902.
Kapic S, Paljetak HC, Alihodzic S, Antolovic R, Haber EV, Jarvest RL, Holmes DJ, Broskey JP, Hunt E. 6-Alkylquinolone-3-carboxylic acid tethered to macrolides synthesis and antimicrobial profile. Bioorg Med Chem. 2010;18:6569–77.
Škugor MM, Štimac V, Palej I, Lugaric D, Paljetak HC, Filic D, Modric M, Ðilovic I, Gembarovski D, Mutak S, Haber VE, Holmes DJ, Ivezic-Schoenfeld Z, Alihodzic S. Synthesis and biological activity of 4”-O-acyl derivatives of 14- and 15-membered macrolides linked to ω-quinolone-carboxylic unit. Bioorg Med Chem. 2010;18:6547–58.
Fajdetic A, Paljetak HC, Lazarevski G, Hutinec A, Alihodzic S, Ðerek M, Štimac V, Andreotti D, Šunjic V, Berge JM, Mutak S, Dumic M, Lociuro S, Holmes DJ, Maršic N, Haber VE, Spaventi R. 4”-O-(ω-Quinolylamino-alkylamino)propionyl derivatives of selected macrolides with the activity against the key erythromycin resistant respiratory pathogens. Bioorg Med Chem. 2010;18:6559–68.
Jakopovic IP, Kragol G, Forrest AK, Frydrych CSV, Štimac V, Kapic S, Škugor MM, Ilijaš M, Paljetak HC, Jelic D, Holmes DJ, Hickey DMB, Verbanac D, Haber VE, Alihodzic S. Synthesis and properties of macrolones characterized by two ether bonds in the linker. Bioorg Med Chem. 2010;18:6578–88.
Kapic S, Fajdetic A, Koštrun S, Cikoš A, Paljetak HC, Antolovic R, Holmes DJ, Alihodzˇic´ S. Synthesis and activity of new macrolones: conjugates between 6(7)-(2’-aminoethyl)-amino-1-cyclopropyl-3-carboxylic acid (2’-hydroxyethyl) amides and 4”-propenoyl-azithromycin. Bioorg Med Chem. 2011;19:7270–80.
Fajdetic A, Vinter A, Paljetak HC, Padovan J, Jakopovic IP, Kapic S, Alihodzic S, Filic D, Modric M, Kosutic-Hulita N, Antolovic R, Schoenfeld ZI, Mutak S, Haber VE, Spaventi R. Synthesis, activity and pharmacokinetics of novel antibacterial 15-membered ring macrolones. Eur J Med Chem. 2011;46:3388–97.
Kapic S, Paljetak HC, Jakopovic IP, Fajdetic A, Ilija M, Štimac V, Brajša K, Holmes DJ, Berge J, Alihodzic S. Synthesis of macrolones with central piperazine ring in the linker and its influence on antibacterial activity. Bioorg Med Chem. 2011;19:7281–98.
Hutinec A, Ðerek M, Lazarevski G, Šunjic´ V, Paljetak HC, Alihodzic S, Haber VE, Dumic M, Maršic´ N, Mutak S. Novel 8a-aza-8a-homoerythromycin-4”-(3-substituted-amino)propionates with broad spectrum antibacterial activity. Bioorg Med Chem Lett. 2010;20:3244–9.
Gaynor M, Mankin AS. Macrolide antibiotics: binding site, mechanism of action, resistance. Curr Top Med Chem. 2003;3:949–61.
Zhang J, Zhu MJ, Lin YN, Zhou HC. The synthesis of benzoxaboroles and their applications in medicinal chemistry. Sci China Chem. 2013;56:1372–81.
Nocentini A, Supuran CT, Winum J-Y. Benzoxaborole compounds for therapeutic uses: a patent review (2010-2018). Expert Opin Ther Pat. 2018;28:493–504.
Printsevskaya SS, Reznikova MI, Korolev AM, Lapa GB, Olsufyeva EN, Preobrazhenskaya MN, Plattner JJ, Zhang YK. Synthesis and study of antibacterial activities of antibacterial glycopeptides antibiotics conjugated with benzoxaboroles. Future Med Chem. 2013;5:641–52.
Qiao ZT, Wang Q, Zhang FL, Wang ZL, Bowling T, Nare B, Jacobs RT, Zhang J, Ding DZ, Liu YG, Zhou HC. Chalcone-benzoxaborole hybrid molecules as potent antitrypanosomal agents. J Med Chem. 2012;55:3553–7.
Tevyashova AN, Korolev AM, Trenin AS, Dezhenkova LG, Shtil AA, Polshakov VI, Savelyev OY, Olsufyeva EN. New conjugates of polyene macrolide amphotericin B with benzoxaboroles: synthesis and properties. J Antibiot. 2016;69:549–60.
Lapa GB, Mirchink EP, Isakova EB, Preobrazhenskaya MN. Two approaches to the use of benzo[c][1,2]oxaboroles as active fragments for synthetic transformation of clarithromycin. J Enzyme Inhib Med Chem 32: 452–6.
Ma S, Jiao B, Liu Z, Wang H, Xian R, Zheng M, Lou H. Synthesis and antibacterial activity of 4”,11-di-O-arylalkylcarbamoyl azithromycin derivatives. Bioorg Med Chem Lett. 2009;19:1698–701.
Osterman IA, Prokhorova IV, Sysoev VO, Boykova YV, Efremenkova OV, Svetlov MS, Kolb VA, Bogdanov AA, Sergiev PV, Dontsova OA. Attenuation-based dual-fluorescent-protein reporter for screening translation inhibitors. Antimicrob Agents Chemother. 2012;56:1774–83.
NCCLS—The National Committee for Laboratory Standards. Performance standards for antimicrobial susceptibility testing. NCCLS document M100-S15, USA, 2005.
Funding
The reported study was partly funded by the Russian Foundation for Basic Research according to the research project no. 16-34-60110 to ANT. Investigation of the mechanism of action of the synthesized compounds was funded by the Russian Science Foundation according to the research project no. 18-44-04005 to IAO.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Tevyashova, A.N., Korolev, A.M., Mirchink, E.P. et al. Synthesis and evaluation of biological activity of benzoxaborole derivatives of azithromycin. J Antibiot 72, 22–33 (2019). https://doi.org/10.1038/s41429-018-0107-2
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41429-018-0107-2
This article is cited by
-
Key areas of antibiotic research conducted at the Gause Institute of New Antibiotics
Russian Chemical Bulletin (2024)
-
Oxaborine Derivatives: Synthesis and Therapeutic Potential
Chemistry of Heterocyclic Compounds (2020)