Abstract
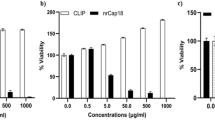
Inhibitors of cancer cell migration and invasion should be useful to inhibit metastasis. Then, we have screened microbial culture filtrates for the inhibitors of cancer cell migration. As a result, we isolated an antibiotic ketomycin from a culture filtrate of Actinomycetes SF2912 as an inhibitor of cancer cell migration. It is a known antibiotic, but its biological activity on mammalian cells has not been reported. Ketomycin inhibited cellular migration and invasion in human breast carcinoma MDA-MB-231 and MCF-7 cells at the non-toxic concentrations. Ketomycin decreased the expressions of MMP-9 and MMP-11 in MDA-MB-231 cells. Knockdown of each gene by siRNA inhibited the cellular migration and invasion. Ketomycin was then found to inhibit the cellular NF-κB activity that may be involved in the upstream signaling. For the mechanism of NF-κB inhibition, ketomycin inhibited autophosphorylation of IKK-α/IKK-β. Ketomycin also inhibited the 3D-invasion of MDA-MB-231 cells at the non-toxic concentrations. Thus, ketomycin having a comparatively simple structure may become a seed of anti-metastasis agent.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Arai Y, et al. Migracins A and B, new inhibitors of cancer cell migration, produced by Streptomyces sp. J Antibiot (Tokyo). 2013;66:225–30.
Ukaji T, Lin Y, Banno K, Okada S, Umezawa K. Inhibition of IGF-1-mediated cellular migration and invasion by migracin A in ovarian clear cell carcinoma cells. PLoS ONE. 2015;10:e0137663.
Sodek KL, Ringuette MJ, Brown TJ. Compact spheroid formation by ovarian cancer cells is associated with contractile behavior and an invasive phenotype. Int J Cancer. 2009;124:2060–70.
Wenzel C, Otto S, Prechtl S, Parczyk K, Steigemann P. A novel 3D high-content assay identifies compounds that prevent fibroblast invasion into tissue surrogates. Exp Cell Res. 2015;339:35–43.
Matsumoto N, et al. Synthesis of NF-κB activation inhibitors derived from epoxyquinomicin C. Bioorg Med Chem Lett. 2000;10:865–69.
Ariga A, Namekawa J, Matsumoto N, Inoue J, Umezawa K. Inhibition of tumor necrosis factor-α-induced nuclear translocation and activation of NF-κB by dehydroxymethylepoxyquinomicin. J Biol Chem. 2002;277:24625–30.
Yamamoto M, Horie R, Takeiri M, Kozawa I, Umezawa K. Inactivation of NF-κB components by covalent binding of (-)-dehydroxymethylepoxyquinomicin to specific cysteine residues. J Med Chem. 2008;51:5780–88.
Takeiri M, et al. Involvement of DNA binding domain in the cellular stability and importin affinity of NF-κB component RelB. Org Biomol Chem. 2012;10:3053–59.
Ukaji T, Lin Y, Okada S, Umezawa K. Inhibition of MMP-2-mediated cellular invasion by NF-κB inhibitor DHMEQ in 3D culture of breast carcinoma MDA-MB-231 cells: a model for early phase of metastasis. Biochem Biophys Res Commun. 2017;485:76–81.
Lin Y, Ukaji T, Koide N, Umezawa K. Inhibition of late and early phases of cancer metastasis by the NF-κB inhibitor DHMEQ derived from microbial bioactive metabolite epoxyquinomicin: a Review. Int J Mol Sci. 2018;19:729 https://doi.org/10.3390/ijms19030729
Saito D, et al. Inhibition of experimental blood-borne lung metastasis by protease inhibitors. Cancer Res. 1980;40:2539–42.
Suzuki K, et al. Combined effect of dehydroxymethylepoxyquinomicin and gemcitabine in a mouse model of liver metastasis of pancreatic cancer. Clin Exp Metastasis. 2013;30:381–92.
Suzuki Y, Sugiyama C, Ohno O, Umezawa K. Preparation and biological activities of optically active dehydroxymethylepoxyquinomicin, a novel NF-κB inhibitor. Tetrahedron. 2004;60:7061–66.
Kang H, et al. N-(4-hydroxyphenyl)retinamide inhibits breast cancer cell invasion through suppressing NF-KB activation and inhibiting matrix metalloproteinase-9 expression. J Cell Biochem. 2012;113:2845–55.
Pires BR, et al. NF-kappaB is involved in the regulation of EMT genes in breast cancer cells. PLoS ONE. 2017;12:e0169622.
Matsumoto G, et al. Targeting of NF-κB pathways by DHMEQ, a novel inhibitor of breast carcinomas: Anti-tumor and anti-angiogenic activity in vivo. Clin Cancer Res. 2005;11:1287–93.
Keller-Schierlein W, Poralla K, Zahner H. Metabolic products of microorganisms 78. isolation, identification and mechanism of action of ketomycin [(R)-3-Cyclohexeneglyoxylic Acid] and of the conversion product 3-cyclohexeneglycine. Arch Mikrobiol. 1969;67:339–56.
Takeda Y, Mak V, Chang CC, Chang CJ, Floss HG. Biosynthesis of ketomycin. J Antibiot. 1984;37:868–75.
Jackson JH, Umbarger HE. Defective transamination, a mechanism for resistance to ketomycin in Escherichia coli. Antimicrob Agents Chemother. 1973;3:510–16.
Acknowledgements
We thank Meiji Seika Pharma Co. Ltd. for the preparation of Actinomycetes culture filtrates. We also thank Drs. Shinichi Kondo and Yoko Ikeda, Bioscience Associates, Tokyo, for the valuable suggestions on isolation and structure determination. This work was financially supported in part by JSPS Kakenhi Grant Number 17K01967, AMED under Grant Number JP18fk0310118, and the Aichi Medical University Research Unit Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
K.U. is supported by Meiji Seika Pharma Co., Ltd, Tokyo, Japan, Shenzhen Wanhe Pharmaceutical Co., Ltd, Shenzhen, China, Fukuyu Hospital, Nisshin, Japan, and Brunese Co., Ltd, Nagoya, Japan. The remaining authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Lin, Y., Chen, Y., Ukaji, T. et al. Isolation of ketomycin from Actinomycetes as an inhibitor of 2D and 3D cancer cell invasion. J Antibiot 72, 148–154 (2019). https://doi.org/10.1038/s41429-018-0129-9
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41429-018-0129-9
This article is cited by
-
Induction of apoptosis and cell cycle arrest in colorectal cancer cells by novel anticancer metabolites of Streptomyces sp. 801
Cancer Cell International (2022)