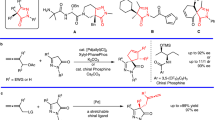
Abstract
A robust synthetic route from l-hydroxyproline (l-Hyp) to phosphines has established an expandable library of six chiral aminophosphines, which were then applied to the phosphine-catalyzed [4 + 2] allene–imine annulation. The enantioinduction in the annulations—induced by a purely steric effect—were moderate (up to 57% ee). A switch of the reaction site from the γ- to the β′-carbon atom of the allenoate was observed during the annulations performed using sterically demanding chiral phosphines.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Vitaku E, Smith DT, Njardarson JT. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J Med Chem. 2014;57:10257–74.
Rubiralta M, Giralt E, Diez A. Piperidine. Structure, preparation, reactivity and synthetic applications of piperidine and its derivatives. Elsevier: Amsterdam; 1991.
Michael JP. Indolizidine and quinolizidine alkaloids. Nat Prod Rep. 2004;21:625–49.
Michael JP. Simple indolizidine alkaloids. In: Cordell GA, editor. The Alkaloids. Vol. 55. San Diego: Academic Press; 2001.
Dewick PM. Medicinal natural products. Chichester: John Wiley and Sons; 1997. Chapter 6.
Buffat MGP. Synthesis of piperidines. Tetrahedron. 2004;60:1701–29.
Zhu X-F, Lan J, Kwon O. An expedient phosphine-catalyzed [4+2] annulation: synthesis of highly functionalized tetrahydropyridines. J Am Chem Soc. 2003;125:4716–7.
Lu K, Kwon O. Phosphine-catalyzed [4+2] annulation: synthesis of ethyl 6-phenyl-tosyl-1,2,5,6-tetrahydropyridine-3-carboxylate. Org Synth. 2009;86:212–24.
Wurz RP, Fu GC. Catalytic asymmetric synthesis of piperidine derivatives through the [4+2] annulation of imines with allenes. J Am Chem Soc. 2005;127:12234–5.
Xiao H, et al. Bifunctional N-acyl-aminophosphine-catalyzed asymmetric [4+2] cycloadditions of allenoates and imines. Chem Eur J. 2011;17:10562–5.
Yu H, et al. Phosphine-catalyzed [4+2] cycloaddition of sulfamate-derived cyclic imines with allenoates: synthesis of sulfamate-fused tetrahydropyridines. Tetrahedron. 2014;70:340–8.
Takizawa S, Arteaga FA, Yoshida Y, Suzuki M, Sasai H. Enantioselective organocatalyzed formal [4+2] cycloaddition of ketimines with allenoates: easy access to a tetrahydropyridine framework with a chiral tetrasubstituted stereogenic carbon center. Asian J. Org. Chem. 2014;3:412–5.
Guo H, Fan YC, Sun Z, Wu Y, Kwon O. Phosphine organocatalysis. Chem Rev. 2018;118:10049–293.
Ni H, Chan W-L, Lu Y. Phosphine-catalyzed asymmetric organic reactions. Chem Rev. 2018;118:9344–411.
Fu W, Tang W. Chiral monophosphorus ligands for asymmetric catalytic reactions. ACS Catal. 2016;6:4814–58.
Tang W, Zhang X. New chiral phosphorus ligands for enantioselective hydrogenation. Chem Rev. 2003;103:3029–70.
Henry CE, et al. Hydroxyproline-derived pseudoenantiomeric [2.2.1] bicyclic phosphines: asymmetric synthesis of (+)- and (−)-pyrrolines. J Am Chem Soc. 2014;136:11890–3.
Smaligo AJ, Vardhineedi S, Kwon O. Carvone-derived P-stereogenic phosphines: design, synthesis, and use in allene–imine [3+2] annulation. ACS Catal. 2018;8:5188–92.
Xu Q, et al. Catalytic enantioselective synthesis of guvacine derivatives through [4 + 2] annulations of imines with α‑methylallenoates. Org Lett. 2018;20:6089–93.
Bertelsen S, Jørgensen KA. Organocatalysis: after the gold rush. Chem Soc Rev. 2009;38:2178–89.
Jensen KL, Dickmeiss G, Jiang H, Albrecht Ł, Jørgensen KA. The diarylprolinol silyl ether system: a general organocatalyst. Acc Chem Res. 2012;45:248–64.
Jiang H, Albrecht Ł, Jørgensen KA. Aminocatalytic remote functionalization strategies. Chem Sci. 2013;4:2287–2300.
Burés J, Armstrong A, Blackmond DG. Curtin–Hammett paradigm for stereocontrol in organocatalysis by diarylprolinol ether catalysts. J Am Chem Soc. 2012;134:6741–50.
Tran YS, Kwon O. Phosphine-catalyzed [4 + 2] annulation: synthesis of cyclohexenes. J Am Chem Soc. 2007;129:12632–3.
Moriarty KJ, et al. N-heterocyclic inhibitors of TNF-ALPHA expression, US2002/65270 (A1); 2002.
Lee K, et al. Melanocortin receptor agonists, US2010120783 (A1); 2010.
Acknowledgements
Financial support for this study was provided by the NIH (R01GM071779). We thank Dr. Saeed Khan (UCLA) for the crystallographic analyses. C.X. thanks Prof. Neil K. Garg and Sarah Anthony (UCLA) for sharing their SFC instrument.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Khong, S.N., Xie, C., Wang, X. et al. Chiral aminophosphines derived from hydroxyproline and their application in allene–imine [4 + 2] annulation. J Antibiot 72, 389–396 (2019). https://doi.org/10.1038/s41429-019-0181-0
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41429-019-0181-0