Abstract
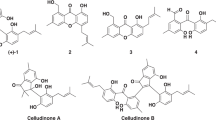
A new drimane sesquiterpene ester, designated insuetusolate (1), and four reported ones (2–5) were isolated from a culture broth of Aspergillus insuetus BF-1613. The chemical structure of 1 was elucidated by extensive spectroscopic analyses, including MS and NMR. Compound 1 has a drimane-type sesquiterpene core with a 2’E,4’E,6’E-octatrienoate side chain. All these drimane sesquiterpene esters inhibited both sterol O-acyl transferase 1 (SOAT1) and 2 (SOAT2), but exhibited slightly potent inhibition against SOAT1 than SOAT2 with selectivity index (SI) [log (IC50 for SOAT1/ IC50 for SOAT2)] values ranging from −0.24 to −0.94.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Rudel LL, Lee RG, Cockman TL. Acyl coenzyme A: cholesterol acyltransferase types 1 and 2: structure and function in atherosclerosis. Curr Opin Lipido. 2001;12:121–7.
Parini P, Davis M, Lada AT, Erickson SK, Wright TL, Gustafsson U, et al. ACAT2 is localized to hepatocytes and is the major cholesterol-esterifying enzyme in human liver. Circulation. 2004;110:2017–23.
Bhattacharjee P, Rutland N, Iyer MR. Targeting Sterol O-Acyltransferase/Acyl-CoA:Cholesterol Acyltransferase (ACAT): a perspective on small-molecule inhibitors and their therapeutic potential. J Med Chem. 2022;65:16062–98.
Ohshiro T, Rudel LL, Omura S, Tomoda H. Selectivity of microbial acyl-CoA: cholesterol acyltransferase inhibitors toward isozymes. J Antibiot. 2007;60:43–51.
Krautbauer S, Weiss TS, Wiest R, Schacherer D, Liebisch G, Buechler C. Diagnostic value of systemic cholesteryl ester/free cholesterol ratio in hepatocellular carcinoma. Anticancer Res. 2017;37:3527–35.
Yue S, Li J, Lee SY, Lee HJ, Shao T, Song B, et al. Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT activation underlies human prostate cancer aggressiveness. Cell Metab. 2014;19:393–406.
Geng F, Cheng X, Wu X, Yoo JY, Cheng C, Guo JY, et al. Inhibition of SOAT1 suppresses glioblastoma growth via blocking SREBP-1-mediated lipogenesis. Clin Cancer Res. 2016;22:5337–48.
Smith DC, Kroiss M, Kebebew E, Habra MA, Chugh R, Schneider BJ, et al. A phase 1 study of nevanimibe HCl, a novel adrenal-specific sterol O-acyltransferase 1 (SOAT1) inhibitor, in adrenocortical carcinoma. Invest N. Drugs. 2020;38:1421–9.
Lada AT, Davis M, Kent C, Chapman J, Tomoda H, Omura S, et al. Identification of ACAT1- and ACAT2-specific inhibitors using a novel, cell-based fluorescence assay: individual ACAT uniqueness. J Lipid Res. 2004;45:378–86.
Sun B-D, Houbraken J, Frisvad J, Jiang X, Chen A, Samson R. New species in Aspergillus section Usti and an overview of Aspergillus section Cavernicolarum. Int J Syst Evol Microbiol. 2020;70:5401–16.
Hu Z, Chen J, Liu Q, Wu Q, Chen S, Wang J, et al. Cyclohexenone derivative and drimane sesquiterpenes from the seagrass-derived fungus Aspergillus insuetus. Chem Biodivers. 2023;20:e202300424.
Gui P, Fan J, Zhu T, Fu P, Hong K, Zhu W. Sesquiterpenoids from the Mangrove-Derived Aspergillus ustus 094102. Mar Drugs. 2022;20:408.
Neuhaus GF, Loesgen S. Antibacterial Drimane Sesquiterpenes from Aspergillus ustus. J Nat Prod. 2021;84:37–45.
Lu Z, Wang Y, Miao C, Liu P, Hong K, Zhu W. Sesquiterpenoids and benzofuranoids from the marine-derived fungus Aspergillus ustus 094102. J Nat Prod. 2009;72:1761–7.
Hayes MA, Wrigley SK, Chetland I, Reynolds EE, Ainsworth AM, Renno DV, et al. Novel drimane sesquiterpene esters from Aspergillus ustus var. pseudodeflectus with endothelin receptor binding activity. J Antibiot. 1996;49:505–12.
Uosaki Y, Yoshida M, Ogawa T, Saitoh Y. RES-1149-1 and -2, novel non-peptidic endothelin type B receptor antagonists produced by Aspergillm sp. J Antibiot. 1996;49:6–12.
Huang Y, Hoefgen S, Valiante V. Biosynthesis of fungal drimane-type sesquiterpene esters. Angew Chem Int Ed. 2021;60:23763–70.
Ohshiro T, Kobayashi K, Ohba M, Matsuda D, Rudel LL, Takahashi T, et al. Selective inhibition of sterolO-acyltransferase 1 isozyme by beauveriolide III in intact cells. Sci Rep. 2017;7:4163.
Ogawa T, Ando K, Tanaka T, Uosaki Y, Matsuda Y. RES-1149-1 and -2, novel non-peptidic endothelin type B receptor antagonists produced by Aspergillus sp. I. Taxonomy of producing strain, fermentation, isolation, and physico-chemical and biological properties. J Antibiot. 1996;49:1–5.
Huang Y, Jin Q, Su M, Ji F, Wang N, Zhong C, et al. Leptin promotes the migration and invasion of breast cancer cells by upregulating ACAT2. Cell Oncol. 2017;40:537–47.
Oni TE, Biffi G, Baker LA, Hao Y, Tonelli C, Somerville TDD, et al. SOAT1 promotes mevalonate pathway dependency in pancreatic cancer. J Exp Med. 2020;217:e20192389.
Jiang Y, Sun A, Zhao Y, Ying W, Sun H, Yang X, et al. Proteomics identifies new therapeutic targets of early-stage hepatocellular carcinoma. Nature. 2019;567:257–61.
Zhu T, Wang Z, Zou T, Xu L, Zhang S, Chen Y et al. SOAT1 Promotes Gastric Cancer Lymph Node Metastasis Through Lipid Synthesis. Front Pharmacol 2021; 12.
Ren M, Xu H, Xia H, Tang Q, Bi F. Simultaneously targeting SOAT1 and CPT1A ameliorates hepatocellular carcinoma by disrupting lipid homeostasis. Cell Death Discov. 2021;7:125.
Acknowledgements
We are grateful to Noriko Sato, Dr. Kenichiro Nagai and Reiko Seki of Graduate School of Pharmaceutical Sciences, Kitasato University for the measurements of NMR and MS spectra and the late Prof. L.L. Rudel of Wake Forest University, Winston-Salem, NC, USA for kindly providing SOAT1-CHO and SOAT2-CHO cells. This work was financially supported by JSPS KAKENHI Grant numbers 18KK0219 (Fund for the Promotion of Joint International Research (Fostering Joint International Research (B)) (HT), MEXT Scholarship (EAAN) and Kitasato University Research Grant for Young Researcher (EAAN).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nur, E.A.A., Kobayashi, K., Tejima, R. et al. Drimane sesquiterpene esters produced by Aspergillus insuetus BF-1613 as inhibitors of sterol O-acyltransferase. J Antibiot 77, 837–841 (2024). https://doi.org/10.1038/s41429-024-00774-8
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41429-024-00774-8
This article is cited by
-
Marinapyrones A and B, new antioxidative α-pyrones produced by soybean rhizosphere-derived actinomycete strain W21-0103
The Journal of Antibiotics (2026)