Abstract
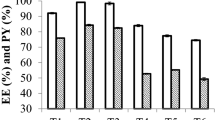
The assignment of new therapeutic purposes to drugs, known as drug repositioning, has been an important ally in the search for new antifungal drugs. Statin compounds, which are used systemically as cholesterol-lowering, may also exert direct antifungal effects, since the statins are drugs that act to prevent sterol synthesis in both humans and fungi and for this reason they are drug promising to combat mycoses. We evaluate the in vivo efficacy of an atorvastatin-loaded topic emulgel (0.75%, 1.5%, or 3.0% m/m) in an in vivo experimental model Tinea pedis. The results showed that the cutaneous delivery-atorvastatin showed total score reduction after seven days of treatment. We concluded that atorvastatin may be a promising drug for the treatment of superficial and cutaneous mycosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Kaitin KI. Clinical pharmacology & therapeutics. Drug Discov Dev. 2010;87:356–61.
Khambhati K, Alessa AH, Singh V. An overview to drug repurposing. Prog Mol Biol Transl Sci. 2025;205:1–8.
Nyilasi I, Kocsubé S, Krizsán K, Galgóczy L, Papp T, Pesti M, et al. Susceptibility of clinically important dermatophytes against statins and different statin-antifungal combinations. Med Mycol. 2014;52:140–8.
González GM, Bonifaz A. Dermatophytosis (Tinea) and other superficial fungal infections. In: Hospenthal DR, Rinaldi MG, Walsh TJ, editors. Diagnosis and treatment of fungal infections. Springer, Cham; 2023.
Costa MC, Pereira de Sá N, Johann S, Santos DA. Social, environmental and microbiologic aspects of endemic mycoses in Brazil. New Microbes N Infect. 2019;29:100496.
Ilkit M, Durdu M. Tinea pedis: The etiology and global epidemiology of a common fungal infection. Crit Rev Microbiol. 2015;41:374–88.
Angely KA, Awa T, Armelle AS, Alain Y, Clemence N, Alain N, et al. Development of dermopharmaceutical forms based on the fruit of Alchornea cordifolia (Euphorbiaceae) for the treatment of dermatophytes. J Adv Pharm Technol Res. 2019;10:143–8.
Ahmady L, Gothwal M, Mukkoli MM, Bari VK. Antifungal drug resistance in Candida: a special emphasis on amphotericin B. APMIS. 2024;132:291–316.
Singh A, Singh PK, de Groot T, Kumar A, Mathur P, Tarai B, et al. Emergence of clonal fluconazole-resistant Candida parapsilosis clinical isolates in a multicentre laboratory-based surveillance study in India. J Antimicrob Chemother. 2019;74:1260–8.
Parent-Michaud M, Dufresne PJ, Fournier E, Folch B, Martineau C, Moreira S, et al. Prevalence and mechanisms of azole resistance in clinical isolates of Aspergillus section Fumigati species in a Canadian tertiary care centre, 2000 to 2013. J Antimicrob Chemother. 2020;75:849–58.
Ghaddar N, Anastasiadis E, Halimeh R, Ghaddar A, Dhar R, AlFouzan W, et al. Prevalence and antifungal susceptibility of Candida albicans causing vaginal discharge among pregnant women in Lebanon. BMC Infect Dis. 2020;20:32.
Gómez-Gaviria M, Contreras-López LM, Aguilera-Domínguez JI, Mora-Montes HM. Strategies of pharmacological repositioning for the treatment of medically relevant mycoses. Infect Drug Resist. 2024;17:2641–58.
Rahal EA, Constantin WN, Zeidan N, Abdelnoor AM. Atorvastatin reduces the survival of Candida albicans-infected BALB/c Mice. Front Microbiol. 2015;6:1474.
Oliveira Neto AS, Souza ILA, Amorim MES, de Freitas Souza T, Rocha VN, do Couto RO, et al. Antifungal efficacy of atorvastatin-containing emulgel in the treatment of oral and vulvovaginal candidiasis. Med Mycol. 2021;59:476–85.
Tavakkoli A, Johnston TP, Sahebkar A. Antifungal effects of statins. Pharmacol Ther. 2020;208:107483.
Mahmoud DE, Faraag AHI, Abu El-Wafa WM. In vitro study on the potential fungicidal effects of atorvastatin in combination with some azole drugs against multidrug resistant Candida albicans. World J Microbiol Biotechnol. 2021;37:191.
Sarita, Mathur N, Chawla P, Mathur M. Repurposing of statins against superficial fungal infections. South East Eur J Public Health. 2024;24:1204–16.
Clinical and Laboratory Standards Institute (CLSI). Reference method for broth dilution antifungal susceptibility testing of yeasts. 4th ed. Wayne, PA: CLSI; 2017. CLSI standard M27.
Cornejo-Garrido J, Salinas-Sandoval M, Díaz-López A, Jácquez-Ríos P, Arriaga-Alba M, Ordaz-Pichardoet C. In vitro and in vivo antifungal activity, liver profile test, and mutagenic activity of five plants used in traditional Mexican medicine. Rev Bras Farmacog. 2015;25:22–8.
Khullar R, Kumar D, Seth N, Saini S. Formulation and evaluation of mefenamic acid emulgel for topical delivery. Saudi Pharm J. 2012;20:63–7.
Fernandes LS, Amorim YM, da Silva EL, Silva SC, Santos AJA, Peixoto FN, et al. Formulation, stability study, and pre-clinical evaluation of a vaginal cream containing curcumin in a rat model of vulvovaginal candidiasis. Mycoses. 2018;61:723–30.
Campos LM, Melo L, Lemos ASO, Guedes MCMR, Silva TP, Figueiredo GF, et al. Mitracarpus frigidus: a promising antifungal in the treatment of vulvovaginal candidiasis. Ind Crop Prod. 2018;123:731–9.
Ajazuddin AA, et al. Recent expansions in an emergent novel drug delivery technology: emulgel. J Control Release. 2013;171:122–32.
Talat M, et al. Emulgel: an effective drug delivery system. Drug Dev Ind Pharm. 2021;47:1193–9.
Vermout S, et al. Pathogenesis of dermatophytosis. Mycopathologia. 2008;166:267–75.
Macreadie IG, Johnson G, Schlosser T, Macreadie PI. Growth inhibition of Candida species and Aspergillus fumigatus by statins. FEMS Microbiol Lett. 2006;262:9–13.
Gupta AK, Ryder JE. Antifungal activity of statins against dermatophytes and Candida species. J Drugs Dermatol. 2003;2:288–91.
Arsic Arsenijevic VS, Milinkovic MM, Vukovic GA. Statins as a new therapy option for fungal infections?. J Chemother. 2017;29:1–9.
Ajdidi A, Sheehan G, Abu Elteen K, Kavanagh K. Assessment of the in vitro and in vivo activity of atorvastatin against Candida albicans. J Med Microbiol. 2019;68:1497–1506.
Acknowledgements
We want to express our gratitude to Colorcon® do Brasil for generously providing the HPMC™ K100 LV used in this research.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Brazil (CAPES)—Finance Code 001 and 88881.709052/2022-01 (PDPG-CONSOLIDAÇÃO-3-4 (PDPG Emergencial de Consolidação Estratégica dos Programas de Pós-graduação (PPGs) stricto sensu acadêmicos com notas 3 e 4).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Oliveira Neto, A.S., Amorim, M.E.S., Fabri, R.L. et al. In vivo efficacy of atorvastatin in the treatment of Tinea pedis: stepping forward into drug repositioning. J Antibiot 78, 600–605 (2025). https://doi.org/10.1038/s41429-025-00848-1
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41429-025-00848-1