Abstract
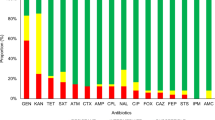
Stressors like translational inhibitors stall protein synthesis and produce a response specific to temperature extremes. Yet, little is known about the expression of temperature-related proteins, particularly the cold shock proteins (Csps), under antibiotic stress. Here, we demonstrate the expression pattern of all nine Csps of Escherichia coli to a sub-lethal concentration of chloramphenicol, tetracycline, gentamicin, kanamycin, and ampicillin. The five antibiotics represent different classes that target different areas of the translational apparatus and the cell wall. To investigate whether all nine csps are expressed in response to antibiotics, we measured the survival of E. coli across the antibiotics and further analyzed expression by qPCR. We find that the expression pattern of csps varies between the Csp groups, and within a Csp group, certain members are more prominently expressed than the rest. The C-group antibiotics, which include chloramphenicol and tetracycline, upregulated the expression of cold-inducible and uncharacterized Csp groups. The H-group antibiotic, kanamycin, along with the uncharacterized antibiotics gentamicin and ampicillin, induced csps as well as heat shock proteins (hsps). To the best of our knowledge, this study is the first to demonstrate the expression pattern of all nine csps in response to antibiotics. Moreover, our study has implications for understanding the triggers of Csps and, in a broader context, their role in stress tolerance, virulence, and pathogenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Michael CA, Dominey-Howes D, Labbate M. The antimicrobial resistance crisis: Causes, consequences, and management. Front Public Heal. 2014;2:1–8.
Dawan J, Ahn J. Bacterial stress responses as potential targets in overcoming antibiotic resistance. Microorganisms. 2022;10:1385.
Al-nabulsi AA, Osaili TM, Shaker RR, Olaimat AN, Jaradat ZW, Zain Elabedeen NA, Holley RA. Effects of osmotic pressure, acid, or cold stresses on antibiotic susceptibility of Listeria monocytogenes. Food Microbiol. 2015;46:154–60.
VanBogelen RA, Neidhardt FC. Ribosomes as sensors of heat and cold shock in Escherichia coli. Proc Natl Acad Sci USA. 1990;87:5589–93.
Cardoso K, Gandra RF, Wisniewski ES, Osaku CA, Kadowaki MK, Felipach-neto V, Haus L, Simão R. DnaK and GroEL are induced in response to antibiotic and heat shock in Acinetobacter baumannii. J Med Microbiol. 2010;59:1061–8.
Jiang W, Jones P, Inouye M. Chloramphenicol induces the transcription of the major cold shock gene of Escherichia coli, cspA. J Bacteriol. 1993;175:5824–8.
Shaw KJ, Miller N, Lerner D, Wan J, Morrow BJ. Comparison of the changes in global gene expression of Escherichia coli induced by four bactericidal agents. J Mol Microbiol Biotechnol. 2003;5:105–22.
Cardoza E, Singh H. Involvement of CspC in response to diverse environmental stressors in Escherichia coli. J Appl Microbiol. 2022;132:785–801.
Muchaamba F, Stephan R. Listeria monocytogenes cold shock proteins: small proteins with a huge impact. Microorganisms. 2021;9:1–20.
Xia B, Ke H, Inouye M. Acquirement of cold sensitivity by quadruple deletion of the cspA family and its suppression by PNPase S1 domain in Escherichia coli. Mol Microbiol. 2001;40:179–88.
Mitta M, Fang L, Inouye M. Deletion analysis of cspA of Escherichia coli: requirement of the AT-rich UP element for cspA transcription and the downstream box in the coding region for its cold shock induction. Mol Microbiol. 1997;26:321–35.
Jiang W, Fang L, Inouye M. The role of the 5’-end untranslated region of the mRNA for CspA, the major cold-shock protein of Escherichia coli, in cold-shock adaptation. J Bacteriol. 1996;178:4919–25.
Yamanaka K, Mitta M, Inouye M. Mutation analysis of the 5’ untranslated region of the cold shock cspA mRNA of Escherichia coli. J Bacteriol. 1999;181:6284–91.
Yamanaka K, Fang L, Inouye M. The CspA family in Escherichia coli: multiple gene duplication for stress adaptation. Mol Microbiol. 1998;27:247–55.
Jiang W, Hou Y, Inouye M. CspA, the major cold-shock Protein of Escherichia coli, Is an RNA Chaperone. J Biol Chem. 1997;272:196–202.
Phadtare S, Inouye M. Role of CspC and CspE in regulation of expression of RpoS and UspA, the stress response proteins in Escherichia coli. J Bacteriol. 2001;183:1205–14.
Shenhar Y, Rasouly A, Biran D, Ron EZ. Adaptation of Escherichia coli to elevated temperatures involves a change in stability of heat shock gene transcripts. Environ Microbiol. 2009;11:2989–97.
Kim Y, Wood TK. Toxins Hha and CspD and Small RNA Regulator Hfq Are Involved in Persister Cell Formation Through MqsR in Escherichia coli. Biochem Biophys Res Commun. 2011;391:209–13.
Scherer S, Neuhaus K Life at low temperatures. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K, Stackebrandt E, editors. The Prokaryotes. Springer, New York, NY; 2006. 210–62.
Westblade LF, Errington J, Dorr T. Antibiotic tolerance. PLoS Pathog. 2020;16:1–7.
Pestka S. Inhibitors of ribosome functions. Annu Rev Microbiol. 1971;25:487–562.
Delhaye A, Collet JF, Laloux G. A fly on the wall: how stress response systems can sense and respond to damage to Peptidoglycan. Front Cell Infect Microbiol. 2019;9:380.
Goltermann L, Good L, Bentin T. Chaperonins Fight Aminoglycoside-induced protein misfolding and promote short-term tolerance in Escherichia coli. J Biol Chem. 2013;288:10483–9.
Stokes JM, Lopatkin AJ, Lobritz MA, Collins JJ. Bacterial metabolism and antibiotic efficacy. Cell Metab. 2019;30:251–9.
Etchegaray JP, Inouye M. CspA, CspB, and CspG, major cold shock proteins of Escherichia coli, are induced at low temperature under conditions that completely block protein synthesis. J Bacteriol. 1999;181:1827–30.
Siibak T, Peil L, Xiong L, Mankin A, Remme J, Tenson T. Erythromycin- and Chloramphenicol-induced ribosomal assembly defects are secondary effects of protein synthesis inhibition. Antimicrob Agents Chemother. 2009;53:563–71.
Graumann P, Marahiel MA. Some like it cold: Response of microorganisms to cold shock. Arch Microbiol. 1996;166:293–300.
Mackow ER, Chang FN. Correlation between RNA synthesis and ppGpp content in Escherichia coli during temperature shifts. MGG Mol Gen Genet. 1983;192:5–9.
Schäfer H, Beckert B, Frese CK, Steinchen W, Nuss AM, Beckstette M, Hantke I, Driller K, Sudzinová P, Krásný L, Kaever V, Dersch P, Bange G, Wilson DN, Turgay K. The alarmones (p) ppGpp are part of the heat shock response of Bacillus subtilis. PLoS Genet. 2020;16:e1008275.
Gallant J, Margason G, Finch B. On the turnover of ppGpp in Escherichia. J Biol Chem. 1972;247:6055–8.
Cardoza E, Singh H. C Group-mediated antibiotic stress mimics the cold shock response. Curr Microbiol. 2021;78:3372–80.
Giuliodori AM, Fabbretti A, Gualerzi C. Cold-responsive regions of paradigm cold-shock and non-cold-shock mRNAs responsible for cold shock translational bias. Int J Mol Sci. 2019;20:457.
Xia B, Ke H, Jiang W, Inouye M. The Cold Box stem-loop proximal to the 5′-end of the Escherichia coli cspA gene stabilizes its mRNA at low temperature. J Biol Chem. 2002;277:6005–11.
Sprengart ML, Fuchs E, Porter AG. The downstream box: An efficient and independent translation initiation signal in Escherichia coli. EMBO J. 1996;15:665–74.
Wang X, Kim Y, Ma Q, Hong SH, Pokusaeva K, Sturino JM, Wood TK. Cryptic prophages help bacteria cope with adverse environments. Nat Commun. 2010;1:147.
Bie L, Zhang M, Wang J, Fang M, Li L, Xu H, Wang M. Comparative analysis of Transcriptomic response of Escherichia coli K-12 MG1655 to nine representative classes of antibiotics. Microbiol Spectr. 2023;11:0031723.
Oughtred R, Rust J, Chang C, Breitkreutz BJ, Stark C, Willems A, Boucher L, Leung G, Kolas N, Zhang F, Dolma S, Coulombe-Huntington J, Chatr-Aryamontri A, Dolinski K, Tyers M. The BioGRID database: A comprehensive biomedical resource of curated protein, genetic, and chemical interactions. Protein Sci. 2021;30:187–200.
Cruz-Loya M, Kang TM, Lozano NA, Watanabe R, Tekin E, Damoiseaux R, Savage VM, Yeh PJ. Stressor interaction networks suggest antibiotic resistance co-opted from stress responses to temperature. ISME J. 2019;13:12–23.
Rodríguez-Verdugo A, Lozano-huntelman N, Cruz-loya M, Savage V, Yeh P. Compounding effects of climate warming and antibiotic resistance. iScience. 2020;23:101024.
Ling J, Cho C, Guo LT, Aerni HR, Rinehart J, Soll D. Protein aggregation caused by aminoglycoside action is prevented by a hydrogen peroxide scavenger. Mol Cell. 2012;48:713–22.
Shenhar Y, Biran D, Ron EZ. Resistance to environmental stress requires the RNA chaperones CspC and CspE. Environ Microbiol Rep. 2012;4:532–9.
Mathieu A, Fleurier S, Frénoy A, Dairou J, Bredeche MF, Sanchez-vizuete P, Song X, Matic I. Discovery and function of a general core hormetic stress response in E. coli induced by sublethal concentrations of antibiotics. Cell Rep. 2016;17:46–57.
Acknowledgements
None.
Author information
Authors and Affiliations
Contributions
Conceptualization, HS.; methodology, EC.; Research and analysis, EC, DV, AD.; writing—original draft preparation, EC, DV, AD.; writing—review and editing, EC, HS.; supervision, EC, HS.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cardoza, E., Vira, D., Rao, A. et al. Altered gene expression of cold shock proteins under antibiotic exposure. J Antibiot 78, 621–632 (2025). https://doi.org/10.1038/s41429-025-00849-0
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41429-025-00849-0