Abstract
Background/objectives
We investigated whether fat mass (FM) and total adipose tissue (TAT) can be used interchangeably and FM per TAT adds to metabolic risk assessment.
Subjects/methods
Cross-sectional data were assessed in 377 adults (aged 18–60 years; 51.2% women). FM was measured by either 4-compartment (4C) model or quantitative magnetic resonance (QMR); total-, subcutaneous- and visceral adipose tissue (TAT, SAT, VAT), and liver fat by whole-body MRI; leptin, insulin, homeostasis model assessment of insulin resistance (HOMA-IR), C-reactive protein (CRP), and triglycerides; resting energy expenditure and respiratory quotient by indirect calorimetry were determined. Correlations and stepwise multivariate regression analyses were performed.
Results
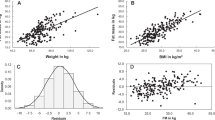
FM4C and FMQMR were associated with TAT (r4C = 0.96, rQMR = 0.99) with a mean FM per TAT of 0.85 and 1.01, respectively. Regardless of adiposity, there was a considerable inter-individual variance of FM/TAT-ratio (FM4C/TAT-ratio: 0.77–0.94; FMQMR/TAT-ratio: 0.89–1.10). Both, FM4C and TAT were associated with metabolic risks. Further, FM4C/TAT-ratio was positively related to leptin but inversely with CRP. There was no association between FM4C/TAT-ratio and VAT/SAT or liver fat. FM4C/TAT-ratio added to the variance of leptin and CRP.
Conclusions
Independent of FM or TAT, FM4C/TAT-ratio adds to metabolic risk assessment. Therefore, the interchangeable use of FM and TAT to assess metabolic risks is questionable as both parameters may complement each other.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Lee SY, Gallagher D. Assessment methods in human body composition. Curr Opin Clin Nutr Metab Care. 2008;11:566–72.
Bosy-Westphal A, Müller MJ. Assessment of fat and lean mass by quantitative magnetic resonance: a future technology of body composition research? Curr Opin Clin Nutr Metab Care. 2015;18:446–51.
Müller MJ, Braun W, Pourhassan M, Geisler C, Bosy-Westphal A. Application of standards and models in body composition analysis. Proc Nutr Soc. 2016;75:181–7.
Ali O, Cerjak D, Kent JW, James R, Blangero J, Zhang Y. Obesity, central adiposity and cardiometabolic risk factors in children and adolescents: a family-based study. Pediatr Obes. 2014;9:e58–62.
Müller MJ, Lagerpusch M, Enderle J, Schautz B, Heller M, Bosy-Westphal A. Beyond the body mass index: tracking body composition in the pathogenesis of obesity and the metabolic syndrome. Obes Rev. 2012;13:6–13.
Kim SK, Kim HJ, Hur KY, Choi SH, Ahn CW, Lim SK, et al. Visceral fat thickness measured by ultrasonography can estimate not only visceral obesity but also risks of cardiovascular and metabolic diseases. Am J Clin Nutr. 2004;79:593–9.
King RJ, Ajjan RA. Vascular risk in obesity: Facts, misconceptions and the unknown. Diab Vasc Dis Res. 2017;14:2–13.
Report of the task group on reference man ICRP Publication 23 (1975). Ann ICRP. 1980;4:III.
Sohlstrom A, Wahlund LO, Forsum E. Adipose tissue distribution as assessed by magnetic resonance imaging and total body fat by magnetic resonance imaging, underwater weighing, and body-water dilution in healthy women. Am J Clin Nutr. 1993;58:830–8.
Wang Z, Zhu S, Wang J, Pierson RNJR, Heymsfield SB. Whole-body skeletal muscle mass: development and validation of total-body potassium prediction models. Am J Clin Nutr. 2003;77:76–82.
Woodard HQ, White DR. The composition of body tissues. Br J Radiol. 1986;59:1209–18.
Choe SS, Huh JY, Hwang IJ, Kim JI, Kim JB. Adipose tissue remodeling: its role in energy metabolism and metabolic disorders. Front Endocrinol. 2016;7:30.
Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–7.
Jernas M, Palming J, Sjoholm K, Jennische E, Svensson P-A, Gabrielsson BG, et al. Separation of human adipocytes by size: hypertrophic fat cells display distinct gene expression. FASEB J. 2006;20:1540–2.
Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673–7.
Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–6.
Pourhassan M, Schautz B, Braun W, Gluer C-C, Bosy-Westphal A, Muller MJ. Impact of body-composition methodology on the composition of weight loss and weight gain. Eur J Clin Nutr. 2013;67:446–54.
Korth O, Bosy-Westphal A, Zschoche P, Glüer CC, Heller M, Müller MJ. Influence of methods used in body composition analysis on the prediction of resting energy expenditure. Eur J Clin Nutr. 2007;61:582–9.
Schoeller DA. Hydrometry. In: Roche AF, Heymsfield SB, Lohman TG, editors. Human body composition. Campaign, IL: Human Kinetics; 1996. pp. 25–43.
Bosy-Westphal A, Schautz B, Later W, Kehayias JJ, Gallagher D, Müller MJ. What makes a BIA equation unique? Validity of eight-electrode multifrequency BIA to estimate body composition in a healthy adult population. Eur J Clin Nutr. 2013;67:S14–21.
Fuller NJ, Jebb SA, Laskey MA, Coward WA, Elia M. Four-component model for the assessment of body composition in humans: Comparison with alternative methods, and evaluation of the density and hydration of fat-free mass. Clin Sci. 1992;82:687–93.
Siervo M, Prado CM, Mire E, Broyles S, Wells JCK, Heymsfield S, et al. Body composition indices of a load-capacity model: gender- and BMI-specific reference curves. Public Health Nutr. 2015;18:1245–54.
Müller MJ, Bosy-Westphal A, Lagerpusch M, Heymsfield SB. Use of balance methods for assessment of short-term changes in body composition. Obesity. 2012;20:701–7.
Müller MJ, Enderle J, Pourhassan M, Braun W, Eggeling B, Lagerpusch M, et al. Metabolic adaptation to caloric restriction and subsequent refeeding: the Minnesota starvation experiment revisited. Am J Clin Nutr. 2015;102:807–19.
Gallagher D, Thornton JC, He Q, Wang J, Yu W, Bradstreet TE, et al. Quantitative magnetic resonance fat measurements in humans correlate with established methods but are biased. Obesity. 2010;18:2047–54.
Bosy-Westphal A, Booke C-A, Blocker T, Kossel E, Goele K, Later W, et al. Measurement site for waist circumference affects its accuracy as an index of visceral and abdominal subcutaneous fat in a Caucasian population. J Nutr. 2010;140:954–61.
Schautz B, Later W, Heller M, Muller MJ, Bosy-Westphal A. Total and regional relationship between lean and fat mass with increasing adiposity—impact for the diagnosis of sarcopenic obesity. Eur J Clin Nutr. 2012;66:1356–61.
Lagerpusch M, Enderle J, Eggeling B, Braun W, Johannsen M, Pape D, et al. Carbohydrate quality and quantity affect glucose and lipid metabolism during weight regain in healthy men. J Nutr. 2013;143:1593–601.
Ma J. Dixon techniques for water and fat imaging. J Magn Reson Imaging. 2008;28:543–58.
Bosy-Westphal A, Kossel E, Goele K, Later W, Hitze B, Settler U, et al. Contribution of individual organ mass loss to weight loss-associated decline in resting energy expenditure. Am J Clin Nutr. 2009;90:993–1001.
Bader N, Bosy-Westphal A, Dilba B, Muller MJ. Intra- and interindividual variability of resting energy expenditure in healthy male subjects—biological and methodological variability of resting energy expenditure. Br J Nutr. 2005;94:843–9.
Ravussin E, Bogardus C. Relationship of genetics, age, and physical fitness to daily energy expenditure and fuel utilization. Am J Clin Nutr. 1989;49:968–75.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9.
Kutner MH, Nachtsheim CJ, Neter J. Applied linear regression models. 4th ed. Boston, MA: McGraw-Hill/Irwin; 2004.
Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57:289–300.
Sims EA. Are there persons who are obese, but metabolically healthy? Metabolism. 2001;50:1499–504.
Lundgren M, Svensson M, Lindmark S, Renstrom F, Ruge T, Eriksson JW. Fat cell enlargement is an independent marker of insulin resistance and ‘hyperleptinaemia’. Diabetologia. 2007;50:625–33.
Ryden M, Andersson DP, Bergstrom IB, Arner P. Adipose tissue and metabolic alterations: regional differences in fat cell size and number matter, but differently: a cross-sectional study. J Clin Endocrinol Metab. 2014;99:E1870–6.
Acknowledgements
This work was supported by the German Research Foundation (DFG MÜ 714/8-3; DFG BO 3296/1-1), the Federal Ministry of Education and Research (BMBF 01EA1336), OMRON, Kyoto, Japan, and Seca GmbH & Co. KG (BCA-01).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Hübers, M., Geisler, C., Bosy-Westphal, A. et al. Association between fat mass, adipose tissue, fat fraction per adipose tissue, and metabolic risks: a cross-sectional study in normal, overweight, and obese adults. Eur J Clin Nutr 73, 62–71 (2019). https://doi.org/10.1038/s41430-018-0150-x
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41430-018-0150-x
This article is cited by
-
Total and regional appendicular skeletal muscle mass prediction from dual-energy X-ray absorptiometry body composition models
Scientific Reports (2023)
-
Smartphone camera based assessment of adiposity: a validation study
npj Digital Medicine (2022)
-
Determinants of ectopic liver fat in metabolic disease
European Journal of Clinical Nutrition (2019)
-
Effect of Over- and Underfeeding on Body Composition and Related Metabolic Functions in Humans
Current Diabetes Reports (2019)