Abstract
Background/Objectives
The escalating obesity epidemic necessitates effective, sustainable weight loss (WL) and maintenance strategies. This study aimed to evaluate the effectiveness of the Weight Loss Maintenance 3 Phases Program (WLM3P) in achieving a clinically significant long-term weight loss (WL) (≥5% initial WL at 18 months) in adults with obesity compared to a standard low-carbohydrate diet (LCD).
Subjects/Methods
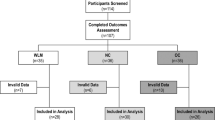
In this two-phase trial, 112 participants targeting initial WL (0–6 months) and subsequent maintenance (7–18 months) were randomly assigned to either WLM3P or LCD groups. Outcomes assessed included change in body weight (kg, %), improvements in body composition, and metabolic profile.
Results
Of 112 randomized participants, 69% (n = 77) completed the study. At 18 months, WL in the WLM3P group (n = 40) was 15.5 ± 8.3% compared to 9.6 ± 8.5% in the LCD group (n = 37) (p < 0.001). The odds ratio of achieving WL ≥ 10% and ≥15% were significantly higher in the WLM3P group. Complete-case analysis revealed significantly greater improvements in BMI, body fat mass, visceral fat area, waist circumference, waist-to-hip ratio, HDL, and triglyceride/HDL ratio in WLM3P than in LCD. No serious adverse events were reported.
Conclusion
Both programs effectively promoted clinically relevant WL and its maintenance. However, the WLM3P program was more successful in helping participants achieve greater WL targets of ≥10% and ≥15%, along with other clinical benefits, after an 18-month intervention.
Trial registration number
NCT04192357.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The full data sets generated during and/or analyzed during the current study are not publicly available because the ethics committee only allowed the use of the data in the context of the present research project; however, anonymized partial data sets or summaries of the data are available from the corresponding author on reasonable request.
References
Frühbeck G, Busetto L, Dicker D, Yumuk V, Goossens GH, Hebebrand J, et al. The ABCD of obesity: an EASO position statement on a diagnostic term with clinical and scientific implications. Obes Facts. 2019;12:131–6. https://doi.org/10.1159/000497124.
Organisation for Economic Cooperation and Development. Obesity among adults. https://www.oecd-ilibrary.org/sites/8cdeadfa-en/index.html?itemId=/content/component/8cdeadfa-en. Accessed 20 Apr 2023.
Instituto Nacional De Estatística. Inquérito Nacional de Saúde 2019. https://www.ine.pt/xportal/xmain?xpid=INE&xpgid=ine_destaques&DESTAQUESdest_boui=414434213&DESTAQUESmodo=2&xlang=pt.
Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. Circulation. 2014;129. https://doi.org/10.1161/01.cir.0000437739.71477.ee.
Durrer Schutz D, Busetto L, Dicker D, Farpour-Lambert N, Pryke R, Toplak H, et al. European practical and patient-centred guidelines for adult obesity management in primary care. Obes Facts. 2019;12:40–66. https://doi.org/10.1159/000496183.
Lv N, Mj Azar K, Rosas LG, Wulfovich S, Xiao L, Ma J, et al. Behavioral lifestyle interventions for moderate and severe obesity: a systematic review. Prev Med. 2017;100:180–93. https://doi.org/10.1016/j.ypmed.2017.04.022.
Soliman, GA. Intermittent fasting and time-restricted eating role in dietary interventions and precision nutrition. Front Public Health. 2022;10. https://doi.org/10.3389/FPUBH.2022.1017254.
Sun JC, Tan ZT, He CJ, Hu HL, Zhai CL, Qian G. Time-restricted eating with calorie restriction on weight loss and cardiometabolic risk: a systematic review and meta-analysis. Eur J Clin Nutr. 2023;77:1014–25. https://doi.org/10.1038/S41430-023-01311-W.
Mah E, Chen O, Liska DJ, Blumberg JB. Dietary supplements for weight management: a narrative review of safety and metabolic health benefits. Nutrients. 2022;14. https://doi.org/10.3390/NU14091787.
Konstantinidi M, Koutelidakis AE. Functional foods and bioactive compounds: a review of its possible role on weight management and obesity’s metabolic consequences. Medicines. 2019;6:94. https://doi.org/10.3390/medicines6030094.
Haghighat N, Ashtary-Larky D, Bagheri R, Wong A, Cheraghloo N, Moradpour G, et al. Effects of 6 months of soy-enriched high protein compared to eucaloric low protein snack replacement on appetite, dietary intake, and body composition in normal-weight obese women: a randomized controlled trial. Nutrients. 2021;13. https://doi.org/10.3390/NU13072266.
Kupila SKE, Joki A, Suojanen LU, Pietiläinen KH. The effectiveness of eHealth interventions for weight loss and weight loss maintenance in adults with overweight or obesity: a systematic review of systematic reviews. Curr Obes Rep. 2023;12:371–94. https://doi.org/10.1007/s13679-023-00515-2.
Irvin L, Madden LA, Marshall P, Vince RV. Digital health solutions for weight loss and obesity: a narrative review. Nutrients. 2023;15. https://doi.org/10.3390/NU15081858.
Dening J, Islam SMS. Defining a low carbohydrate diet: proposal for a standardized consensus of carbohydrate intake (Carb-Cal Model). Diabetes Res Clin Pract. 2020;166. https://doi.org/10.1016/J.DIABRES.2020.108284.
Clifton PM, Condo D, Keogh JB. Long term weight maintenance after advice to consume low carbohydrate, higher protein diets-a systematic review and meta analysis. Nutr Metab Cardiovasc Dis. 2014;24:224–35. https://doi.org/10.1016/J.NUMECD.2013.11.006.
Lei L, Huang J, Zhang L, Hong Y, Hui S, Yang J. Effects of low-carbohydrate diets versus low-fat diets on metabolic risk factors in overweight and obese adults: a meta-analysis of randomized controlled trials. Front Nutr. 2022;9. https://doi.org/10.3389/FNUT.2022.935234/FULL.
Magkos F. Protein-rich diets for weight loss maintenance. Curr Obes Rep. 2020;9:213–8. https://doi.org/10.1007/S13679-020-00391-0.
Moon J, Koh G. Clinical evidence and mechanisms of high-protein diet-induced weight loss. J Obes Metab Syndr. 2020;29:166. https://doi.org/10.7570/JOMES20028.
Direção-Geral da Saúde. Avaliação Antropométrica No Adulto. Orientação no 017/2013 de 05/12/2013. 2013:1–9.
Direção-Geral de Saúde. Norma nº 026/2011 – Abordagem Terapêutica da Hipertensão Arterial (atualizada 19/03/2013). Lisboa: Direção-Geral da Saúde; 2013.
Martins I, Porto A. e Oliveira L. Tabela de composição de alimentos. INSA. 2006
Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International Physical Activity Questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–95. https://doi.org/10.1249/01.MSS.0000078924.61453.FB.
Garaulet M, Ortega FB, Ruiz JR, Rey-López JP, Béghin L, Manios Y, et al. Short sleep duration is associated with increased obesity markers in European adolescents: effect of physical activity and dietary habits. The HELENA Study. Int J Obes. 2011;35:1308–17. https://doi.org/10.1038/IJO.2011.149.
Vijayananthan A, Nawawi O. The importance of good clinical practice guidelines and its role in clinical trials. Biomed Imaging Interv J. 2008;4:1–4. https://doi.org/10.2349/biij.4.1.e5.
Acharya SD, Elci OU, Sereika SM, Music E, Styn MA, Turk MW, et al. Adherence to a behavioral weight loss treatment program enhances weight loss and improvements in biomarkers. Patient Prefer Adherence. 2009;3:151. https://doi.org/10.2147/PPA.S5802.
Nelson M, Atkinson M, Darbyshire S. Food Photography II: portion size and the nutrient content of meals. Br J Nutr. 1996;76:31–49.
Horn DB, Almandoz JP, Look M. What is clinically relevant weight loss for your patients and how can it be achieved? A narrative review. Postgrad Med. 2022;134:359–75. https://doi.org/10.1080/00325481.2022.2051366.
Li G, Rios RS, Wang XX, Yu Y, Zheng KI, Huang OY, et al. Sex influences the association between appendicular skeletal muscle mass to visceral fat area ratio and non-alcoholic steatohepatitis in patients with biopsy-proven non-alcoholic fatty liver disease. Br J Nutr. 2022;127:1613–20. https://doi.org/10.1017/S0007114521002415.
Liu D, Zhong J, Wen W, Ruan Y, Zhang Z, Sun J, et al. Relationship between skeletal muscle mass to visceral fat area ratio and cardiovascular risk in type 2 diabetes. Diabetes Metab Syndr Obes. 2021;14:3733–42. https://doi.org/10.2147/DMSO.S326195.
Liang S, Mijatovic J, Li A, Koemel N, Nasir R, Toniutti C, et al. Dietary patterns and non-communicable disease biomarkers: a network meta-analysis and nutritional geometry approach. Nutrients. 2023;15. https://doi.org/10.3390/NU15010076/S1.
Raynor HA, Looney SM, Steeves EA, Spence M, Gorin AA. The effects of an energy density prescription on diet quality and weight loss: a pilot randomized controlled trial. J Acad Nutr Diet. 2012;112:1397–402. https://doi.org/10.1016/J.JAND.2012.02.020.
Lemstra M, Bird Y, Nwankwo C, Rogers M, Moraros J. Weight loss intervention adherence and factors promoting adherence: a meta-analysis. Patient Prefer Adherence. 2016;10:1547–59. https://doi.org/10.2147/PPA.S103649.
Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, et al. National sleep foundation’s updated sleep duration recommendations: final report. Sleep Health. 2015;1:233–43. https://doi.org/10.1016/J.SLEH.2015.10.004.
Gudzune KA, Doshi RS, Mehta AK, Chaudhry ZW, Jacobs DK, Vakil RM, et al. Efficacy of commercial weight-loss programs: an updated systematic review. Ann Intern Med. 2015;162:501–12. https://doi.org/10.7326/M14-2238.
Carneiro DM, Freire RC, Honório TCDD, Zoghaib I, Cardoso FFDSES, Tresvenzol LMF, et al. Randomized, double-blind clinical trial to assess the acute diuretic effect of Equisetum arvense (field horsetail) in healthy volunteers. Evid Based Complement Alternat Med. 2014;2014:760683 https://doi.org/10.1155/2014/760683.
Elobeid MA, Padilla MA, McVie T, Thomas O, Brock DW, Musser B, et al. Missing data in randomized clinical trials for weight loss: scope of the problem, state of the field, and performance of statistical methods. PLoS ONE. 2009;4:e6624. https://doi.org/10.1371/JOURNAL.PONE.0006624.
Lopes C, Torres D, Oliveira A, Severo M, Alarcão V, Guiomar S, et al. Inquérito Alimentar Nacional e de Atividade Física, IAN-AF 2015-2016: Relatório de resultados. 2016 p. 1–291.
Acknowledgements
The authors express their gratitude to all participants involved in this study.
Funding
This work was sponsored by Farmodiética S.A. The study was also promoted by the CINTESIS@RISE, NOVA Medical School, NOVA University of Lisbon and financed by national funds through FCT Fundação para a Ciência e a Technologia, I.P., within the scope of the project “RISE – LA/P/0053/2020”. The sponsor and funder had no role in the study design and had no role during its execution, analysis, interpretation of the data, or decision to submit the results.
Author information
Authors and Affiliations
Contributions
André Moreira-Rosário, Conceição Calhau, Diana Teixeira, Filipa Cortez, Marta P. Silvestre, and Vanessa Pereira conceived the study design. André Moreira-Rosário and Marta P. Silvestre are the methodology and clinical nutrition leaders, respectively; in turn, Conceição Calhau is the principal investigator of this clinical trial. Vanessa Pereira was responsible for the protocol and procedures writing under the supervision of André Moreira-Rosário and Marta P. Silvestre. Vanessa Pereira drafted the manuscript. Cláudia Camila Dias was responsible for the statistical analysis plan and did not have any interference in the study design and implementation. Inês Barreiros-Mota, Conceição Calhau, Diana Teixeira, Filipa Cortez, Cláudia Camila Dias, André Moreira-Rosário, and Marta P. Silvestre reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
Vanessa Pereira and Filipa Cortez work at Farmodiética S.A., one of the study sponsors. The sponsor provided support in the form of salaries for the author (Vanessa Pereira) and research materials but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the paper. The other researchers do not have any relationship with this sponsor.
Ethics approval and consent to participate
This trial was registered at clinical trials.gov under the identifier NCT04192357. The recruitment and data collection of this study have already been completed. The study was approved by the Ethics Committee of the NOVA Medical School (Lisbon, Portugal) (CEFCM Approval Number: 108/2018) and was registered at www.clinicaltrials.gov (NCT04192357) before participants’ recruitment. All participants provided written informed consent in accordance with the principles of the Declaration of Helsinki. Written informed consent has been obtained from the patients to publish this paper.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pereira, V., Barreiros-Mota, I., Cortez, F. et al. A randomized controlled trial of a weight loss maintenance program in adults with obesity: the WLM3P study. Eur J Clin Nutr 78, 694–702 (2024). https://doi.org/10.1038/s41430-024-01454-4
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41430-024-01454-4