Abstract
Background
This study examined (1) associations between sociodemographic and clinical variables with low muscle mass and radiodensity and their loss relative to treatment commencement in patients with lung cancer; and (2) the magnitude of change in muscle mass and association with treatment outcomes and survival.
Methods
Prospective study in patients planned for curative (chemo)radiotherapy for lung cancer. Low skeletal muscle mass and radiodensity and muscle loss were determined from pre- and post-treatment computed tomography images. Sociodemographic, clinical, functional, nutritional, physical activity and alternate body composition were assessed pre-treatment. Logistic and linear regression and Fisher’s exact tests were used to assess associations between variables and study outcomes. Cox proportional hazards models were fitted to examine associations with survival.
Results
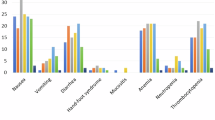
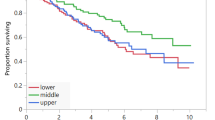
Overall, 53 patients (62.3% male) with a mean age of 69 ± 9.3 years and 54.8% with stage III disease were included. Pre-treatment low calf circumference was associated with pre-treatment low muscle mass (p = 0.006). Higher comorbidity scores pre-treatment were associated with normal muscle radiodensity pre- and post-treatment (p = 0.015, p = 0.027, respectively). Pre-treatment low energy and protein intake were associated with low muscle radiodensity post-treatment. Muscle mass and radiodensity were not associated with survival or treatment outcomes.
Conclusions
In patients with lung cancer, there is some evidence anthropometric measures of muscle mass are suggestive of low muscle mass pre-radiotherapy, while low energy intake pre-treatment may indicate low muscle radiodensity after treatment. However, these findings are limited by the small sample size and further prospective studies with larger samples are required.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The data generated and analysed during this study can be found within the published article and its supplementary files.
References
International Agency for Research on Cancer. Globocan World Fact Sheet: World Health Organisation; 2021 Available from: https://www.google.com/search?client=firefox-b-d&q=globocan.
Baracos V, Reiman T, Mourtzakis M, Gioulbasanis I, Antoun S. Body composition in patients with non-small cell lung cancer: a contemporary view of cancer cachexia with the use of computed tomography image analysis. Am J Clin Nutr. 2010;91:1133S–7S.
Cederholm T, Jensen GL, Correia M, Gonzalez MC, Fukushima R, Higashiguchi T, et al. GLIM criteria for the diagnosis of malnutrition—a consensus report from the global clinical nutrition community. J Cachexia Sarcopenia Muscle. 2019;10:207–17.
Lin T-Y, Chen Y-F, Wu W-T, Han D-S, Tsai IC, Chang K-V, et al. Impact of sarcopenia on the prognosis and treatment of lung cancer: an umbrella review. Discov Oncol. 2022;13:115.
Kiss NK, Denehy L, Edbrooke L, Prado CM, Ball D, Siva S, et al. Predicting muscle loss during lung cancer treatment (PREDICT): protocol for a mixed methods prospective study. BMJ Open. 2021;11:e051665.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83.
Prado CMM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9:629–35.
Martin L, Birdsell L, MacDonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31:1539–47.
Ottery F. Definition of standardized nutritional assessment and interventional pathways in oncology. Nutrition. 1996;12:S15–S9.
Malmstrom TK, Morley JE. SARC-F: a simple questionnaire to rapidly diagnose sarcopenia. J Am Med Dir Assoc. 2013;14:531–2.
Kaysen GA, Zhu F, Sarkar S, Heymsfield SB, Wong J, Kaitwatcharachai C, et al. Estimation of total-body and limb muscle mass in hemodialysis patients by using multifrequency bioimpedance spectroscopy2. Am J Clin Nutr. 2005;82:988–95.
Schectman O, Sindhu BS. Grip assessment. In: American Society of Hand Therapists, editor. Clinical assessment recommendations. Third ed. Mount Laurel, NJ: American Society of Hand Therapists; 2015.
Simonsick EM, Guralnik JM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94.
Gonzalez MC, Mehrnezhad A, Razaviarab N, Barbosa-Silva TG, Heymsfield SB. Calf circumference: cutoff values from the NHANES 1999-2006. Am J Clin Nutr. 2021;113:1679–87.
National Cancer Institute. Dietary Assessment Primer: National Cancer Institute; Available from: https://dietassessmentprimer.cancer.gov/profiles/table.html.
Thompson FE, Subar AF. Dietary assessment methodology. In: Coulston AM, Boushey CJ, Ferruzzi MG, Delahanty LM, editors. Nutrition in the prevention and treatment of disease (Fourth Edition): Academic Press; 2017.
National Institutes of Health, National Cancer Institute. Division of Cancer Control & Population Sciences ASA24-Australia [August 2023]. Available from: https://epi.grants.cancer.gov/asa24/respondent/australia.html.
Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–95.
Butt Z, Webster K, Eisenstein A, Beaumont J, Eton DT, Masters G, et al. Quality of life in lung cancer: the validity and cross-cultural applicability of the functional assessment of cancer therapy—lung scale. Hematol Oncol Clin North Am. 2005;19:389–420.
Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, et al. The functional assessment of cancer therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–9.
Asaad M, Habibullah NK, Butler CE. The impact of COVID-19 on clinical trials. Ann Surg. 2020;272:e222–e3.
Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge MP, Albu J, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol 2004;97:2333–8.
Ganesh S, Cave V. P-values, p-values everywhere! NZ Vet J. 2018;66:55–6.
Oflazoglu U, Alacacioglu A, Varol U, Kucukzeybek Y, Salman T, Taskaynatan H, et al. Prevalence and related factors of sarcopenia in newly diagnosed cancer patients. Support Care Cancer. 2020;28:837–43.
Xiao J, Caan BJ, Cespedes Feliciano EM, Meyerhardt JA, Kroenke CH, Baracos VE, et al. The association of medical and demographic characteristics with sarcopenia and low muscle radiodensity in patients with nonmetastatic colorectal cancer. Am J Clin Nutr. 2019;109:615–25.
Xiao J, Caan BJ, Weltzien E, Cespedes Feliciano EM, Kroenke CH, Meyerhardt JA, et al. Associations of pre-existing co-morbidities with skeletal muscle mass and radiodensity in patients with non-metastatic colorectal cancer. J Cachexia Sarcopenia Muscle. 2018;9:654–63.
Barazzoni R, Jensen GL, Correia M, Gonzalez MC, Higashiguchi T, Shi HP, et al. Guidance for assessment of the muscle mass phenotypic criterion for the Global Leadership Initiative on Malnutrition (GLIM) diagnosis of malnutrition. Clin Nutr. 2022;41:1425–33.
Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36:11–48.
Kiss N, Loeliger J, Findlay M, Isenring E, Baguley BJ, Boltong A, et al. Clinical oncology society of Australia: position statement on cancer-related malnutrition and sarcopenia. Nutr Diet. 2020;77:416–25.
Erul E, Guven DC, Onur MR, Yazici G, Aksoy S. Role of sarcopenia on survival and treatment-related toxicity in head and neck cancer: a narrative review of current evidence and future perspectives. Eur Arch Otorhinolaryngol. 2023;280:3541–56.
Erul E, Guven DC, Ozbay Y, Altunbulak AY, Kahvecioglu A, Ercan F, et al. Evaluation of sarcopenia as a prognostic biomarker in locally advanced head and neck squamous cell carcinoma. Biomark Med. 2023;17:87–99.
Acknowledgements
The authors acknowledge the research assistants and clinicians who supported the recruitment of participants to the PREDICT study and access to the CT images.
Funding
NK and this study are supported by a Victorian Cancer Agency Nursing and Allied Health Clinical Research Fellowship (grant no: CRFNAH18001).
Author information
Authors and Affiliations
Contributions
Conceptualisation: NK, GA, CMP, RMD, LD, DB, LE, SS, SFF, AU, NH, AW, GW. Data curation: ARC, NK, NH, AW, GW, AL, AC. Formal analysis: NK, GA, ARC. Writing—original draft preparation: NK. Writing—reviewing and editing: all authors.
Corresponding author
Ethics declarations
Competing interests
NK reports honoraria from Abbott Nutrition Australasia. CP reports honoraria or consulting fees from Abbott Nutrition, Nestle Health Sciences, Nutricia and Pfizer. RD reports honoraria from Abbott Nutrition Australasia and Fresenius Kabi. GA, ARC, LD, LE, DB, SS, SFF, AU, NH, AW, GW, AL and AC have no conflict of interest to declare.
Ethical approval and consent to participate
This study received ethics approval from the Human Research Ethics Committee at Peter MacCallum Cancer Centre (HREC/53147/PMCC-2019) and Deakin University (2019-320). All methods were performed in accordance with the Declaration of Helsinki and relevant ethical guidelines at Peter MacCallum Cancer Centre and Deakin University and written informed consent was obtained from all participants.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kiss, N., Prado, C.M., Abbott, G. et al. Sociodemographic and clinical factors associated with low muscle mass and composition in people treated with (chemo)radiotherapy for lung cancer. Eur J Clin Nutr 79, 369–378 (2025). https://doi.org/10.1038/s41430-024-01552-3
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41430-024-01552-3