Abstract
Objectives
The purpose of this study was to compare selected iron markers (serum iron, total iron-binding capacity (TIBC), transferrin and ferritin concentration, transferrin saturation, and free haemoglobin) in children from marginalised Roma communities (MRCs) with children from the majority population and explore their associations with diet composition.
Methods
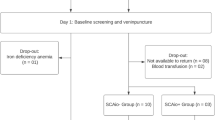
We obtained cross-sectional data (questionnaires, blood samples from children) from 119 mother-child dyads from MRCs and the majority population. Group differences were tested using Chi-square and Mann–Whitney U tests. Associations of belonging to MRCs and diet with iron markers (transferrin, ferritin, TIBC, serum iron, transferrin saturation) were examined using bootstrapped linear regression models, and mediation analyses assessed whether eating habits mediated group differences.
Results
Statistically significant differences between children from MRCs, and the majority were found in serum transferrin, ferritin, and TIBC levels. The more frequent consumption of sweetened drinks, sweets, and salty snacks is associated with lower levels of transferrin, and more frequent consumption of dairy products is associated with higher levels of total iron-binding capacity. Current breastfeeding was found to be negatively associated with ferritin. Consumption of sweets and salty snacks partially mediates the differences in transferrin between children from MRCs and the majority.
Conclusions
Our findings suggest that the observed low ferritin levels, elevated TIBC, and reduced transferrin saturation in Roma children are likely indicative of early-stage iron deficiency, potentially driven by underlying malnutrition. This study underscores the significant disparities in iron metabolism between children from MRCs and those from the majority population, primarily driven by social determinants of health, including diet composition.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Data described in the manuscript will be made available upon request to the corresponding author.
References
FRA – European Union Agency for Fundamental Rights. Roma in 10 European Countries - Main results | European Union Agency for Fundamental Rights. Vienna, https://doi.org/10.2811/221064.2022
Office of the Plenipotentiary of the Government of the Slovak Republic for Roma Communities, Office of the Government of the Slovak Republic. Atlas rómskych komunít 2019 | Úrad splnomocnenca vlády SR pre rómske komunity. 2019.https://www.romovia.vlada.gov.sk/atlas-romskych-komunit/atlas-romskych-komunit-2019/ (accessed 1 Sep 2025).s
Victora CG, Hartwig FP, Vidaletti LP, Martorell R, Osmond C, Richter LM, et al. Effects of early-life poverty on health and human capital in children and adolescents: analyses of national surveys and birth cohort studies in LMICs. Lancet. 2022;399:1741–52.
Shonkoff JP, Garner AS, Siegel BS, Dobbins MI, Earls MF, McGuinn L, et al. The lifelong effects of early childhood adversity and toxic stress. Pediatrics 129. https://doi.org/10.1542/PEDS.2011-2663.2012;
Lee EJ, Keyes K, Bitfoi A, Mihova Z, Pez O, Yoon E, et al. Mental health disparities between Roma and non-Roma children in Romania and Bulgaria. BMC Psychiatry. 2014;14:1–7.
Guerrero Z, Civišová D, Winkler P. Mental health and access to care among the Roma population in Europe: A scoping review. Transcult Psychiatry. 2024;61:118–30.
Djurovic D, Prcic S, Milojkovic M, Konstantinidis G, Tamburlini G. The health status of Roma children -A medical or social issue?. Eur Rev Med Pharmacol Sci. 2014;18:1218–1223.
Vresk L, Flanagan M, Daniel AI, Potani I, Bourdon C, Spiegel-Feld C et al. Micronutrient status in children aged 6-59 months with severe wasting and/or nutritional edema: implications for nutritional rehabilitation formulations. Nutr Rev. https://doi.org/10.1093/NUTRIT/NUAD165. 2024.
Espinosa-Salas S, Gonzalez-Arias M Nutrition: Micronutrient Intake, Imbalances, and Interventions. 2023.
Mondon C, Tan PY, Chan CL, Tran TN, Gong YY. Prevalence, determinants, intervention strategies and current gaps in addressing childhood malnutrition in Vietnam: a systematic review. BMC Public Health. 2024;24:1–39.
Gallagher PG. Anemia in the pediatric patient. Blood. 2022;140:571–93.
Letuka T, Frade S. Household and individual risk factors of anaemia among under-5 children in Lesotho. Afr Health Sci. 2020;20:1478.
Amoah WW, Kobi D, Tabong PT-N, Kukeba MW, Alhassan Y, Achaliwie F et al. Factors Contributing to Malnutrition among Children Under 5 Years at St. Elizabeth Catholic Hospital, Ahafo Hwidiem. Clin Med Insights Pediatr 18. https://doi.org/10.1177/11795565231222716. 2024.
Ara G, Fawad B, Shabbir S Malnutrition in children under five years in a squatter settlement of Karachi: a case-control study. BMC Public Health 24. https://doi.org/10.1186/S12889-024-18359-3. 2024.
Begna G, Bikila H, Biru B, Diriba D, Tolera C, Dessalegn R et al. Determinants of severe acute malnutrition among children less than five years visiting health centers in Leqa Dulacha District, East Wallaga Zone, Oromia Region, Ethiopia: A case control study. Health Sci Rep 7.https://doi.org/10.1002/HSR2.1939.2024;
Balendran S, Forsyth C. Non-anaemic iron deficiency. Aust Prescr. 2021;44:193.
Levels and trends in child malnutrition: UNICEF/WHO/The World Bank Group joint child malnutrition estimates: key findings of the 2021 edition. https://www.who.int/publications/i/item/9789240025257 (accessed 5 Aug 2024).
Giampaolo R, Marotta R, Biagiarelli FS, Zampa A, Moramarco S, Buonomo E The exacerbated prevalence of acute malnutrition and growth retardation in Roma children living in camps. Ital J Pediatr 47. https://doi.org/10.1186/S13052-021-01122-4. 2021.
Puig S, Ramos-Alonso L, Romero AM, Martínez-Pastor MT. The elemental role of iron in DNA synthesis and repair. Metallomics. 2017;9:1483–1500.
Bonsu EO, Addo IY, Boadi C, Boadu EF, Okeke SR Determinants of iron-rich food deficiency among children under 5 years in sub-Saharan Africa: a comprehensive analysis of Demographic and Health Surveys. BMJ Open 14. https://doi.org/10.1136/BMJOPEN-2023-079856,. 2024.
Du Y, Liao Y, Leng F, Li L, Ye R, Mao Y, et al. Anaemia prevalence and its associated factors in children under 5 years in Western China: A systematic review. BMJ Paediatr Open 6. https://doi.org/10.1136/BMJPO-2021-001185 2022.
Survey on Income and Living Conditions of Households in Marginalised Roma Communities - in connection with the Atlas of Roma communities | European Union Agency for Fundamental Rights. https://fra.europa.eu/en/promising-practices/survey-income-and-living-conditions-households-marginalised-roma-communities (accessed 1 Sep 2025).
Tran TD, Luchters S, Fisher J. Early childhood development: impact of national human development, family poverty, parenting practices and access to early childhood education. Child Care Health Dev. 2017;43:415–26.
Luby J, Belden A, Botteron K, Marrus N, Harms MP, Babb C, et al. The effects of poverty on childhood brain development: the mediating effect of caregiving and stressful life events. JAMA Pediatr. 2013;167:1135–42.
Soans JS, Noronha JA, Mundkur SC, Nayak BS, Garg M, Jathanna RD, et al. Mapping evidence on the impact of junk food on anaemia among adolescent and adult population: a scoping review. BMC Nutr. 2025;11:1–20.
Učme sa učiť sa by ETP Slovensko - Issuu. https://issuu.com/etpslovensko/docs/ucme-sa-ucit-sa (accessed 1 Sep 2025).
Shonkoff JP. Building a new biodevelopmental framework to guide the future of early childhood policy. Child Dev. 2010;81:357–67.
Solar O, Irwin A A conceptual framework for action on the social determinants of health. Social Determinants of Health Discussion Paper 2 (Policy and Practice). World Heal. WHO Press: Geneva, 2010.
Auerbach M, Deloughery TG, Tirnauer JS. Iron deficiency in adults: a review. JAMA. 2025;333:1813–23.
Gkouvatsos K, Papanikolaou G, Pantopoulos K. Regulation of iron transport and the role of transferrin. Biochim Biophys Acta Gen Subj. 2012;1820:188–202.
Miniero R, Talarico V, Galati MC, Giancotti L, Saracco P, Raiola G Iron Deficiency and Iron Deficiency Anemia in Children. In: Rodrigo L (ed). Iron Deficiency Anemia. IntechOpen: Rijeka, 2018 https://doi.org/10.5772/intechopen.79790.
Gattermann N, Muckenthaler MU, Kulozik AE, Metzgeroth G, Hastka J. Investigation of Iron Deficiency and Iron Overload. Dtsch Arztebl Int. 2021;118:847–56.
Seiden J, Waldman M, Mccoy DC, Fink G Data Management & Scoring Manual. 2021.
Čvorović J Paternal investment, stepfather presence and early child development and growth among Serbian Roma. Evol Hum Sci 4. https://doi.org/10.1017/EHS.2022.14.2022;
He L, Guo C, Su Y, Ding N The relationship between serum ferritin level and clinical outcomes in sepsis based on a large public database. Sci Rep https://doi.org/10.1038/s41598-023-35874-2.2023.
Drvenica IT, Stančić AZ, Maslovarić IS, Trivanović DI, Ilić VL. Extracellular Hemoglobin: Modulation of Cellular Functions and Pathophysiological Effects. Biomolecules. 2022;12:1708.
Gecková AM, Babinská I, Bobáková D, Veselská Z, Bosáková L, Kolarčik P, et al. Socioeconomic characteristics of the population living in Roma settlements and their association with health and health-related behaviour. Cent Eur J Public Health. 2014;22:S57–S64.
Dale JC, Burritt MF, Zinsmeister AR. Diurnal variation of serum iron, iron-binding capacity, transferrin saturation, and ferritin levels. Am J Clin Pathol. 2002;117:802–8.
Kundrapu S, Noguez J Laboratory Assessment of Anemia. In: Makowski GS (ed). Advances in Clinical Chemistry. Elsevier, pp 197–225.2018,
Wang AYM Nutrition and anemia in chronic kidney disease. In: Kopple JD, Massry SG, Kamyar K-Z, Fouque D (eds). Nutritional Management of Renal Disease. Academic Press, pp 741–760.2022,
Kant AK. Consumption of energy-dense, nutrient-poor foods by adult Americans: Nutritional and health implications. The third National Health and Nutrition Examination Survey, 1988–94. Am J Clin Nutr. 2000;72:929–36.
Webb KL, Lahti-Koski M, Rutishauser I, Hector DJ, Knezevic N, Gill T, et al. Consumption of ‘extra’ foods (energy-dense, nutrient-poor) among children aged 16-24 months from western Sydney, Australia. Public Health Nutr. 2006;9:1035–44.
Van Der Merwe LF, Eussen SR Iron status of young children in Europe. In: Am J Clin Nutr. https://doi.org/10.3945/ajcn.117.156018. 2017.
Higgins V, Chan MK, Adeli K. Pediatric Reference Intervals for Transferrin Saturation in the CALIPER Cohort of Healthy Children and Adolescents. EJIFCC. 2017;28:77.
Balázs P, Rákóczi I, Grenczer A, Foley KL. Birth-weight differences of Roma and non-Roma neonates - public health implications from a population-based study in Hungary. Cent Eur J Public Health. 2014;22:24–28.
Nikolić TK, Ponikvar BM, Zakrajšek V Differences in preterm births and birth weight between new-borns of roma and non-roma mothers in slovenia. Popul Med 5. https://doi.org/10.18332/POPMED/164062. 2023.
Dharod JM, Frazier CM, Labban J, Black MM Breastfeeding duration and associations with prevention of accelerated growth among infants from low-income, racially and ethnically diverse backgrounds. Public Health Nutr 27. https://doi.org/10.1017/S1368980023002689,. 2024.
Ruangkit C, Prachakittikul N, Hemprachitchai N, Dumrongwongsiri O, Soonsawad S Association of infant feeding practices with iron status and hematologic parameters in 6-month-old infants. Children 8. https://doi.org/10.3390/CHILDREN8121159,. 2021.
Chen CM, Mu SC, Shih CK, Chen YL, Tsai LY, Kuo YT, et al. Iron status of infants in the first year of life in northern Taiwan. Nutrients 12. https://doi.org/10.3390/NU12010139,. 2020.
Liao SL, Yao TC, Hua MC, Tsai MH, Hsu SY, Chen LC, et al. Trajectory of vitamin D, micronutrient status and childhood growth in exclusively breastfed children. Sci Rep 9. https://doi.org/10.1038/S41598-019-55341-1 2019.
Basrowi RW, Zulfiqqar A, Sitorus NL. Anemia in Breastfeeding Women and Its Impact on Offspring’s Health in Indonesia: A Narrative Review. Nutrients. 2024;16:1285.
Dumrongwongsiri O, Winichagoon P, Chongviriyaphan N, Suthutvoravut U, Grote V, Koletzko B. Zinc and iron adequacy and relative importance of zinc/iron storage and intakes among breastfed infants. Matern Child Nutr. 2021;18:e13268.
Csölle I, Felső R, Szabó É, Metzendorf MI, Schwingshackl L, Ferenci T, et al. Health outcomes associated with micronutrient-fortified complementary foods in infants and young children aged 6–23 months: a systematic review and meta-analysis. Lancet Child Adolesc Health. 2022;6:533–44.
Aksu T, Ünal Ş. Iron Deficiency Anemia in Infancy, Childhood, and Adolescence. Turkish Archives of Pediatrics. https://doi.org/10.5152/TurkArchPediatr.2023.23049. 2023.
Graczykowska K, Kaczmarek J, Wilczyńska D, Łoś-Rycharska E, Krogulska A. The consequence of excessive consumption of cow’s milk: Protein-losing enteropathy with anasarca in the course of iron deficiency anemia—case reports and a literature review. Nutrients. 2021;13:1–14.
Zikidou P, Tsigalou C, Trypsianis G, Karvelas A, Tsalkidis A, Mantadakis E. Prevalence of Anemia, Iron Deficiency, Iron Deficiency Anemia and Diagnostic Performance of Hematologic and Biochemical Markers of Sideropenia in 1- to 5-Year-Old Children in Thrace Greece. Mediterr J Hematol Infect Dis. 2022;14:e2022054.
Soppi ET. Iron deficiency without anemia – a clinical challenge. Clin Case Rep. 2018;6:1082–6.
Thorisdottir AV, Ramel A, Palsson GI, Tomassson H, Thorsdottir I. Iron status of one-year-olds and association with breast milk, cow’s milk or formula in late infancy. Eur J Nutr. 2013;52:1661–8.
Funding
This research was funded by the Slovak Research and Development Agency under Contract No. APVV-19-0493 and from ERDF/ESF project DigiWELL (No. CZ.02.01.01/00/22_008/0004583).
Author information
Authors and Affiliations
Contributions
DFB and BH conceptualised the study and developed the methodology. BH and JM conducted the formal analysis. The investigation was carried out by DFB, BH, JM, IV, BČ, and AB. BH and JM were responsible for data curation. BH and JM prepared the original draft. DFB, MZ, and IV contributed to review and editing of the manuscript. Visualisation and supervision were provided by DFB. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving research study participants were approved by the Ethics Committees in both the Presov (No. 03682/2022/OZ-20) and the Kosice (“RomaREACH”) regions and by the Ethics Committee of the Medical Faculty at P.J. Safarik University in Kosice (16 N/2021). Written informed consent was obtained from all participants (or their legal guardians) involved in the study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hubková, B., Mašlanková, J., Večurkovská, I. et al. Disturbed homeostasis of iron metabolism in children from marginalised Roma communities. Eur J Clin Nutr (2025). https://doi.org/10.1038/s41430-025-01686-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41430-025-01686-y