Abstract
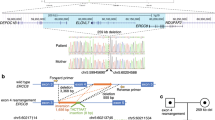
Cockayne syndrome is an autosomal recessive multisystem disorder characterized by intellectual disability, microcephaly, severe growth failure, sensory impairment, peripheral neuropathy, and cutaneous sensitivity. This rare disease is linked to disease-causing variations in the ERCC6 (CSB) and ERCC8 (CSA) genes. Various degrees of severity have been described according to age at onset and survival, without any clear genotype-phenotype correlation. All types of nucleotide changes have been observed in CS genes, including splice variations mainly affecting the splice site consensus sequences. We report here the case of two brothers from a consanguineous family presenting a severe but long-term survival phenotype of Cockayne syndrome. We identified in the patients a homozygous deep intronic nucleotide variation causing the insertion of a cryptic exon in the ERCC8 (CSA) transcript, by modifying intronic regulatory elements important for exon definition. The pathogenesis of the nucleotide variant NG_009289.1(NM_000082.3):c.173+1119G>C was validated in vitro with a reporter minigene system. To our knowledge, these are the first Cockayne patients described with this kind of disease-causing variation, though molecular mechanism underlying early onset symptoms and unexpected slow raise of progression of the disease remain to be elucidated.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Kleijer WJ, Laugel V, Berneburg M, et al. Incidence of DNA repair deficiency disorders in western Europe: Xeroderma pigmentosum, Cockayne syndrome and trichothiodystrophy. DNA Repair. 2008;7:744–50.
Laugel V. Cockayne syndrome: the expanding clinical and mutational spectrum. Mech Ageing Dev. 2013;134:161–70.
Wilson BT, Stark Z, Sutton RE, et al. The Cockayne Syndrome Natural History (CoSyNH) study: clinical findings in 102 individuals and recommendations for care. Genet Med. 2016;18:483–93.
Mayne LV, Lehmann AR. Failure of RNA synthesis to recover after UV irradiation: an early defect in cells from individuals with Cockayne’s syndrome and xeroderma pigmentosum. Cancer Res. 1982;42:1473–8.
Schmickel RD, Chu EH, Trosko JE, Chang CC. Cockayne syndrome: a cellular sensitivity to ultraviolet light. Pediatrics. 1977;60:135–9.
Henning KA, Li L, Iyer N, et al. TheCockayne syndrome group A gene encodes a WD repeat protein that interacts with CSB protein and a subunit of RNA polymerase II TFIIH. Cell. 1995;82:555–64.
Troelstra C, van Gool A, de Wit J, Vermeulen W, Bootsma D, Hoeijmakers JH. ERCC6, a member of a subfamily of putative helicases, is involved in Cockayne’s syndrome and preferential repair of active genes. Cell. 1992;71:939–53.
Karikkineth AC, Scheibye-Knudsen M, Fivenson E, Croteau DL, Bohr VA. Cockayne syndrome: clinical features, model systems and pathways. Ageing Res Rev. 2017;33:3–17.
Groisman R, Kuraoka I, Chevallier O, et al. CSA-dependent degradation of CSB by the ubiquitin–proteasome pathway establishes a link between complementation factors of the Cockayne syndrome. Genes Dev. 2006;20:1429–34.
Wang Y, Chakravarty P, Ranes M, et al. Dysregulation of gene expression as a cause of Cockayne syndrome neurological disease. Proc Natl Acad Sci. 2014;111:14454–9.
Laugel V, Dalloz C, Durand M, et al. Mutation update for the CSB/ERCC6 and CSA/ERCC8 genes involved in Cockayne syndrome. Hum Mutat. 2010;31:113–26.
Calmels N, Greff G, Obringer C, et al. Uncommon nucleotide excision repair phenotypes revealed by targeted high-throughput sequencing. Orphanet J Rare Dis. 2016;11:26.
Laugel V, Dalloz C, Stary A, et al. Deletion of 5′ sequences of the CSB gene provides insight into the pathophysiology of Cockayne syndrome. Eur J Hum Genet. 2008;16:320–7.
Yeo G, Burge CB. Maximum entropy modeling of short sequence motifs with applications to RNA splicing signals. J Comput Biol J Comput Mol Cell Biol. 2004;11:377–94.
Reese MG, Eeckman FH, Kulp D, Haussler D. Improved splice site detection in genie. J Comput Biol. 1997;4:311–23.
Desmet F-O, Hamroun D, Lalande M, Collod-Beroud G, Claustres M, Beroud C. Human splicing finder: an online bioinformatics tool to predict splicing signals. Nucleic Acids Res. 2009;37:e67–e67.
Geoffroy V, Pizot C, Redin C, et al. VaRank: a simple and powerful tool for ranking genetic variants. PeerJ. 2015;3:e796.
Lek M, Karczewski KJ, Minikel EV, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–91.
Burn TC, Connors TD, Klinger KW, Landes GM. Increased exon-trapping efficiency through modifications to the pSPL3 splicing vector. Gene. 1995;161:183–7.
Houdayer C, Caux-Moncoutier V, Krieger S, et al. Guidelines for splicing analysis in molecular diagnosis derived from a set of 327 combined in silico/in vitro studies on BRCA1 and BRCA2 variants. Hum Mutat. 2012;33:1228–38.
Chasin LA. Searching for splicing motifs. Adv Exp Med Biol. 2007;623:85–106.
Wang Z, Burge CB. Splicing regulation: from a parts list of regulatory elements to an integrated splicing code. RNA. 2008;14:802–13.
Ishii S, Nakao S, Minamikawa-Tachino R, Desnick RJ, Fan J-Q. Alternative splicing in the α-galactosidase a gene: increased exon inclusion results in the fabry cardiac phenotype. Am J Hum Genet. 2002;70:994–1002.
King K, Flinter F, Nihalani V, Green P. Unusual deep intronic mutations in the COL4A5 gene cause X linked Alport syndrome. Hum Genet. 2002;111:548–54.
Davis RL, Homer VM, George PM, Brennan SO. A deep intronic mutation in FGB creates a consensus exonic splicing enhancer motif that results in afibrinogenemia caused by aberrant mRNA splicing, which can be corrected in vitro with antisense oligonucleotide treatment. Hum Mutat. 2009;30:221–7.
Homolova K, Zavadakova P, Doktor TK, Schroeder LD, Kozich V, Andresen BS. The deep intronic c.903+469T>C mutation in the MTRR gene creates an SF2/ASF binding exonic splicing enhancer, which leads to pseudoexon activation and causes the cblE type of homocystinuria. Hum Mutat. 2010;31:437–44.
Trabelsi M, Beugnet C, Deburgrave N, et al. When a mid-intronic variation of DMD gene creates an ESE site. Neuromuscul Disord. 2014;24:1111–7.
Mallery DL, Tanganelli B, Colella S, et al. Molecular analysis of mutations in the CSB (ERCC6) gene in patients with Cockayne syndrome. Am J Hum Genet. 1998;62:77–85.
Komatsu A, Suzuki S, Inagaki T, Yamashita K, Hashizume K. A kindred with Cockayne syndrome caused by multiple splicing variants of theCSA gene. Am J Med Genet. 2004;128A:67–71.
Acknowledgments
We would like to thank the family involved in this study. We also thank Dr Amelie Piton for providing pSPL3B vector and for scientific discussions. We also thank Dr Jean Muller for bioinformatic advice and Mrs. Sinthuja Pachchek for bioinformatic support in exome sequencing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they no competing interest.
Rights and permissions
About this article
Cite this article
Schalk, A., Greff, G., Drouot, N. et al. Deep intronic variation in splicing regulatory element of the ERCC8 gene associated with severe but long-term survival Cockayne syndrome. Eur J Hum Genet 26, 527–536 (2018). https://doi.org/10.1038/s41431-017-0009-y
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41431-017-0009-y
This article is cited by
-
Heterogeneous clinical features in Cockayne syndrome patients and siblings carrying the same CSA mutations
Orphanet Journal of Rare Diseases (2022)