Abstract
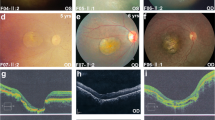
Cone and cone-rod dystrophies (CD and CRD, respectively) are degenerative retinal diseases that predominantly affect the cone photoreceptors. The underlying disease gene is not known in approximately 75% of autosomal recessive cases. Variants in NMNAT1 cause a severe, early-onset retinal dystrophy called Leber congenital amaurosis (LCA). We report two patients where clinical phenotyping indicated diagnoses of CD and CRD, respectively. NMNAT1 variants were identified, with Case 1 showing an extremely rare homozygous variant c.[271G > A] p.(Glu91Lys) and Case 2 compound heterozygous variants c.[53 A > G];[769G > A] p.(Asn18Ser);(Glu257Lys). The detailed variant analysis, in combination with the observation of an associated macular atrophy phenotype, indicated that these variants were disease-causing. This report demonstrates that the variants in NMNAT1 may cause CD or CRD associated with macular atrophy. Genetic investigations of the patients with CD or CRD should include NMNAT1 in the genes examined.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Roosing S, Thiadens AA, Hoyng CB, Klaver CC, den Hollander AI, Cremers FP. Causes and consequences of inherited cone disorders. Prog Retin Eye Res. 2014;42:1–26.
Michaelides M, Moore AT. The cone dysfunction syndromes. Br J Ophthalmol. 2004;88:291–7.
Nash BM, Wright DC, Grigg JR, Bennetts B, Jamieson RV. Retinal dystrophies, genomic applications in diagnosis and prospects for therapy. Transl Pediatr. 2015;4:139–63.
Chiang PW, Wang J, Chen Y, et al. Exome sequencing identifies NMNAT1 mutations as a cause of Leber congenital amaurosis. Nat Genet. 2012;44:972–4.
Koenekoop RK, Wang H, Majewski J, et al. Mutations in NMNAT1 cause Leber congenital amaurosis and identify a new disease pathway for retinal degeneration. Nat Genet. 2012;44:1035–9.
Perrault I, Hanein S, Zanlonghi X, et al. Mutations in NMNAT1 cause Leber congenital amaurosis with early-onset severe macular and optic atrophy. Nat Genet. 2012;44:975–7.
Falk MJ, Zhang Q, Nakamaru-Ogiso E, et al. NMNAT1 mutations cause Leber congenital amaurosis. Nat Genet. 2012;44:1040–5.
Sasaki Y, Margolin Z, Borgo B, Havranek JJ, Milbrandt J. Characterization of Leber congenital amaurosis-associated NMNAT1 mutants. J Biol Chem. 2015;290:17228–38.
Ma AS, Grigg JR, Ho G, et al. Sporadic and familial congenital cataracts: mutational spectrum and new diagnoses using next-generation sequencing. Hum Mutat. 2016;37:371–84.
Richards A, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association of Molecular Pathology. Genet Med. 2015;17:405–24.
Venselaar H, Te Beek TA, Kuipers RK, Hekkelman ML, Vriend G. Protein structure analysis of mutations causing inheritable diseases. An e-science approach with life scientist friendly interfaces. BMC Bioinformatics. 2010;11:548.
Jin X, Qu LH, Meng XH, Xu HW, Yin ZQ. Detecting genetic variations in hereditary retinal dystrophies with next-generation sequencing technology. Mol Vis. 2014;20:553–60.
Siemiatkowska AM, van den Born LI, van Genderen MM, et al. Novel compound heterozygous NMNAT1 variants associated with Leber congenital amaurosis. Mol Vis. 2014;20:753–9.
El-Haig WM, Jakobsson C, Favez T, Schorderet DF, Abouzeid H. Novel ADAM9 homozygous mutation in a consanguineous Egyptian family with severe cone-rod dystrophy and cataract. Br J Ophthalmol. 2014;98:1718–23.
Kamenarova K, Corton M, Garcia-Sandoval B, et al. Novel GUCA1A mutations suggesting possible mechanisms of pathogenesis in cone, cone-rod, and macular dystrophy patients. Biomed Res Int. 2013;2013:517570.
Kiernan DF, Shah RJ, Hariprasad SM, et al. Thirty-year follow-up of an African American family with macular dystrophy of the retina, locus 1 (North Carolina macular dystrophy). Ophthalmology. 2011;118:1435–43.
Ajmal M, Khan MI, Neveling K, et al. A missense mutation in the splicing factor gene DHX38 is associated with early-onset retinitis pigmentosa with macular coloboma. J Med Genet. 2014;51:444–8.
Smith D, Oestreicher J, Musarella MA. Clinical spectrum of leber’s congenital amaurosis in the second to fourth decades of life. Ophthalmology. 1990;97:1156–61.
Aboshiha J, Dubis AM, van der Spuy J, et al. Preserved outer retina in AIPL1 Leber’s congenital amaurosis: implications for gene therapy. Ophthalmology. 2015;122:862–4.
Siemiatkowska AM, Schuurs-Hoeijmakers JH, Bosch DG, et al. Nonpenetrance of the most frequent autosomal recessive leber congenital amaurosis mutation in NMNAT1. JAMA Ophthalmol. 2014;132:1002–4.
Khan AO, Budde BS, Nurnberg P, Kawalia A, Lenzner S, Bolz HJ. Genome-wide linkage and sequence analysis challenge CCDC66 as a human retinal dystrophy candidate gene and support a distinct NMNAT1-related fundus phenotype. Clin Genet. 2017. https://doi.org/10.1111/cge.13022
Acknowledgements
We would like to thank the families for their willingness to participate in this study. This study was supported by the National Health and Medical Research Council of Australia (NHMRC Grant: 1099165 to R.V.J. and J.R.G.) and the Costco Organisation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Benjamin M. Nash and Richard Symes contributed equally to this work.
Rights and permissions
About this article
Cite this article
Nash, B.M., Symes, R., Goel, H. et al. NMNAT1 variants cause cone and cone-rod dystrophy. Eur J Hum Genet 26, 428–433 (2018). https://doi.org/10.1038/s41431-017-0029-7
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41431-017-0029-7