Abstract
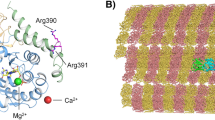
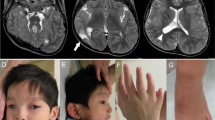
ARL13B encodes for the ADP-ribosylation factor-like 13B GTPase, which is required for normal cilia structure and Sonic hedgehog (Shh) signaling. Disruptions in cilia structure or function lead to a class of human disorders called ciliopathies. Joubert syndrome is characterized by a wide spectrum of symptoms, including a variable degree of intellectual disability, ataxia, and ocular abnormalities. Here we report a novel homozygous missense variant c.[223G>A] (p.(Gly75Arg) in the ARL13B gene, which was identified by whole-exome sequencing of a trio from a consanguineous family with multiple-affected individuals suffering from intellectual disability, ataxia, ocular defects, and epilepsy. The same variant was also identified in a second family. We saw a striking difference in the severity of ataxia between affected male and female individuals in both families. Both ARL13B and ARL13B-c.[223G>A] (p.(Gly75Arg) expression rescued the cilia length and Shh defects displayed by Arl13b hennin (null) cells, indicating that the variant did not disrupt either ARL13B function. In contrast, ARL13B-c.[223G>A] (p.(Gly75Arg) displayed a marked loss of ARL3 guanine nucleotide-exchange factor activity, with retention of its GTPase activities, highlighting the correlation between its loss of function as an ARL3 guanine nucleotide-exchange factor and Joubert syndrome.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Cantagrel V, Silhavy JL, Bielas SL, et al Mutations in the cilia gene ARL13B lead to the classical form of Joubert syndrome. Am J Hum Genet. 2008;83:170–9.
Larkins CE, Aviles GD, East MP, Kahn RA, Caspary T. Arl13b regulates ciliogenesis and the dynamic localization of Shh signaling proteins. Mol Biol Cell. 2011;22:4694–703.
Thomas S, Cantagrel V, Mariani L, et al Identification of a novel ARL13B variant in a Joubert syndrome-affected patient with retinal impairment and obesity. Eur J Hum Genet. 2015;23:621–7.
Lu H, Toh MT, Narasimhan V, Thamilselvam SK, Choksi SP, Roy S. A function for the Joubert syndrome protein Arl13b in ciliary membrane extension and ciliary length regulation. Dev Biol. 2015;397:225–36.
Gotthardt K, Lokaj M, Koerner C, Falk N, Giessl A, Wittinghofer A. A G-protein activation cascade from Arl13B to Arl3 and implications for ciliary targeting of lipidated proteins. Elife. 2015;4:e11859.
Caspary T, Larkins CE, Anderson KV. The graded response to Sonic Hedgehog depends on cilia architecture. Dev Cell. 2007;12:767–78.
Higginbotham H, Guo J, Yokota Y, et al Arl13b-regulated cilia activities are essential for polarized radial glial scaffold formation. Nat Neurosci. 2013;16:1000–7.
Kasahara K, Miyoshi K, Murakami S, Miyazaki I, Asanuma M. Visualization of astrocytic primary cilia in the mouse brain by immunofluorescent analysis using the cilia marker arl13b. Acta Med Okayama. 2014;68:317–22.
Higginbotham H, Eom TY, Mariani LE, et al Arl13b in primary cilia regulates the migration and placement of interneurons in the developing cerebral cortex. Dev Cell. 2012;23:925–38.
Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129:865–77.
Cherfils J, Zeghouf M. Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol Rev. 2013;93:269–309.
Doherty D. Joubert syndrome: insights into brain development, cilium biology, and complex disease. Semin Pediatr Neurol. 2009;16:143–54.
Romani M, Micalizzi A, Valente EM. Joubert syndrome: congenital cerebellar ataxia with the molar tooth. Lancet Neurol. 2013;12:894–905.
Gleeson JG, Keeler LC, Parisi MA, et al. Molar tooth sign of the midbrain-hindbrain junction: occurrence in multiple distinct syndromes. Am J Med Genet A. 2004;125A:125–34
Gorden NT, Arts HH, Parisi MA, et al CC2D2A is mutated in Joubert syndrome and interacts with the ciliopathy-associated basal body protein CEP290. Am J Hum Genet. 2008;83:559–71.
Bachmann-Gagescu R, Dempsey JC, Phelps IG, et al Joubert syndrome: a model for untangling recessive disorders with extreme genetic heterogeneity. J Med Genet. 2015;52:514–22.
Kang HG, Lee HK, Ahn YH, et al Targeted exome sequencing resolves allelic and the genetic heterogeneity in the genetic diagnosis of nephronophthisis-related ciliopathy. Exp Mol Med. 2016;48:e251.
Shaheen R, Szymanska K, Basu B, et al Characterizing the morbid genome of ciliopathies. Genome Biol. 2016;17:242.
Rafiullah R, Aslamkhan M, Paramasivam N, et al Homozygous missense mutation in the LMAN2L gene segregates with intellectual disability in a large consanguineous Pakistani family. J Med Genet. 2016;53:138–44.
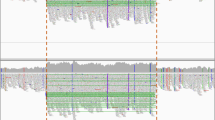
Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–60.
Li H, Handsaker B, Wysoker A, et al The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25:2078–9.
Rimmer A, Phan H, Mathieson I, et al Integrating mapping-, assembly- and haplotype-based approaches for calling variants in clinical sequencing applications. Nat Genet. 2014;46:912–8.
Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38:e164.
Liu X, Jian X, Boerwinkle E. dbNSFP: a lightweight database of human nonsynonymous SNPs and their functional predictions. Hum Mutat. 2011;32:894–9.
Miertzschke M, Koerner C, Spoerner M, Wittinghofer A. Structural insights into the small G-protein Arl13B and implications for Joubert syndrome. Biochem J. 2014;457:301–11.
Eswar N, Webb B, Marti-Renom MA et al. Comparative protein structure modeling using MODELLER. Curr Protoc Protein Sci. 2007;Chapter 2:Unit 2 9.
Sali A, Blundell TL. Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol. 1993;234:779–815.
John B, Sali A. Comparative protein structure modeling by iterative alignment, model building and model assessment. Nucleic Acids Res. 2003;31:3982–92.
Mariani LE, Bijlsma MF, Ivanova AI, Suciu SK, Kahn RA, Caspary T. Arl13b regulates Shh signaling from both inside and outside the cilium. Mol Biol Cell. 2016;27:3780–90.
Schindelin J, Arganda-Carreras I, Frise E, et al Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–82.
Cooper MK, Porter JA, Young KE, Beachy PA. Teratogen-mediated inhibition of target tissue response to Shh signaling. Science. 1998;280:1603–7.
Taipale J, Chen JK, Cooper MK, et al Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature. 2000;406:1005–9.
Bowzard JB, Sharer JD, Kahn RA. Assays used in the analysis of Arl2 and its binding partners. Methods Enzymol. 2005;404:453–67.
Bowzard JB, Cheng D, Peng J, Kahn RA. ELMOD2 is an Arl2 GTPase-activating protein that also acts on Arfs. J Biol Chem. 2007;282:17568–80.
Cavenagh MM, Breiner M, Schurmann A, et al ADP-ribosylation factor (ARF)-like 3, a new member of the ARF family of GTP-binding proteins cloned from human and rat tissues. J Biol Chem. 1994;269:18937–42.
Saleheen D, Natarajan P, Armean IM, et al Human knockouts and phenotypic analysis in a cohort with a high rate of consanguinity. Nature. 2017;544:235–9.
Lek M, Karczewski KJ, Minikel EV, et al Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–91.
Ivanova AA, East MP, Yi SL, Kahn RA. Characterization of recombinant ELMOD (cell engulfment and motility domain) proteins as GTPase-activating proteins (GAPs) for ARF family GTPases. J Biol Chem. 2014;289:11111–21.
Hori Y, Kobayashi T, Kikko Y, Kontani K, Katada T. Domain architecture of the atypical Arf-family GTPase Arl13b involved in cilia formation. Biochem Biophys Res Commun. 2008;373:119–24.
Randazzo PA, Terui T, Sturch S, Fales HM, Ferrige AG, Kahn RA. The myristoylated amino terminus of ADP-ribosylation factor 1 is a phospholipid- and GTP-sensitive switch. J Biol Chem. 1995;270:14809–15.
Amor JC, Horton JR, Zhu X, et al Structures of yeast ARF2 and ARL1: distinct roles for the N terminus in the structure and function of ARF family GTPases. J Biol Chem. 2001;276:42477–84.
Seidel RD 3rd, Amor JC, Kahn RA, Prestegard JH. Conformational changes in human Arf1 on nucleotide exchange and deletion of membrane-binding elements. J Biol Chem. 2004;279:48307–18.
Ivanova AA, Caspary T, Seyfried NT et al. Biochemical characterization of purified mammalian ARL13B indicate that it is an atypical GTPase and ARL3 guanine nucleotide exchange factor (GEF). J Biol Chem. 2017;292:11091–108.
Baron-Cohen S, Knickmeyer RC, Belmonte MK. Sex differences in the brain: implications for explaining autism. Science. 2005;310:819–23.
Acknowledgments
We thank the families for participating and supporting this study. We also thank Dr. Ute Hehr (Center for Human Genetics, Regensburg, Germany) for her opinion on the CT scans. We thank Cheryl Timms Strauss for editing of the manuscript. We also thank Christine Fischer for statistical advice.
Funding
This study was supported by the Medical Faculty of Heidelberg (R.R., S.B., and G.A.R.), the National Institutes of Health (GM110663 to T.C., A.B.L., A.A.I., and R.A.K.), and the Emory Integrated Genomics Core (EIGC), which is subsidized by the Emory University School of Medicine. R.R. was supported by a scholarship from the German Academic Exchange Service (DAAD; 91541533) and G.M. and R.C.W. gratefully acknowledge the support of the Klaus Tschira Foundation.
Author contributions
R.R. performed the genetic analysis, expression analysis in mice and SH-SY5Y cells, and data analysis. A.B.L. and T.C. performed and analyzed the data in Arl13b hnn MEFs. A.A.I. and R.A.K. performed and analyzed the GAP and GEF assays. H.A. evaluated the patients, clinical data, and CT scans. S.B. contributed to the data interpretation. N.P., M.S., and S.W. performed and analyzed WES data. G.M. and R.C.W. performed and analyzed the 3D structure modeling. E.B. interpreted the CT scans of the patients. G.A.R. initiated, supervised, and supported the project. R.R., S.B., R.K., T.C., and G.A.R. wrote the manuscript. All authors commented on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Patient consent
Obtained.
Ethical approval
This study was approved by the Institutional Ethical Review Committee, University of Health Sciences Lahore, Pakistan, and the Ethikkommission, Medical Faculty Heidelberg, Germany (S-035/2014).
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Rafiullah, R., Long, A.B., Ivanova, A.A. et al. A novel homozygous ARL13B variant in patients with Joubert syndrome impairs its guanine nucleotide-exchange factor activity. Eur J Hum Genet 25, 1324–1334 (2017). https://doi.org/10.1038/s41431-017-0031-0
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41431-017-0031-0
This article is cited by
-
Exome sequencing reveals broad genetic heterogeneity for neuromuscular disorders in consanguineous Pakistani Families
European Journal of Human Genetics (2025)
-
Primary cilia signaling in astrocytes mediates development and regional-specific functional specification
Nature Neuroscience (2024)