Abstract
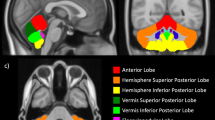
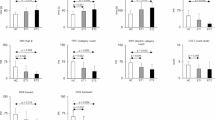
Celia’s encephalopathy (progressive encephalopathy with/without lipodystrophy, PELD) is a recessive neurodegenerative disease that is fatal in childhood. It is caused by a c.985C>T variant in the BSCL2/seipin gene that results in an aberrant seipin protein. We evaluated neurological development before and during treatment with human recombinant leptin (metreleptin) plus a dietary intervention rich in polyunsaturated fatty acids (PUFA) in the only living patient. A 7 years and 10 months old girl affected by PELD was treated at age 3 years with metreleptin, adding at age 6 omega-3 fatty acid supplementation. Her mental age was evaluated using the Battelle Developmental Inventory Screening Test (BDI), and brain PET/MRI was performed before treatment and at age 5, 6.5, and 7.5 years. At age 7.5 years, the girl remains alive and leads a normal life for her mental age of 30 months, which increased by 4 months over the last 18 months according to BDI. PET images showed improved glucose uptake in the thalami, cerebellum, and brainstem. This patient showed a clear slowdown in neurological regression during leptin replacement plus a high PUFA diet. The aberrant BSCL2 transcript was overexpressed in SH-SY5Y cells and was treated with docosahexaenoic acid (200 µM) plus leptin (0.001 mg/ml) for 24 h. The relative expression of aberrant BSCL2 transcript was measured by qPCR. In vitro studies showed significant reduction (32%) in aberrant transcript expression. This therapeutic approach should be further studied in this devastating disease.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Guillen-Navarro E, Sanchez-Iglesias S, Domingo-Jimenez R, et al. A new seipin-associated neurodegenerative syndrome. J Med Genet. 2013;50:401–9.
Alaei MR, Talebi S, Ghofrani M, Taghizadeh M, Keramatipour M. Whole exome sequencing reveals a BSCL2 mutation causing progressive encephalopathy with lipodystrophy (PELD) in an Iranian pediatric patient. Iran Biomed J. 2016;20:295–301.
Ruiz-Riquelme A, Sánchez-Iglesias S, Rábano A, et al. Larger aggregates of mutant seipin in Celia’s Encephalopathy, a new protein misfolding neurodegenerative disease. Neurobiol Dis. 2015;83:44–53.
Sanchez-Iglesias S, Unruh-Pinheiro A, Guillin-Amarelle C, et al. Skipped BSCL2 transcript in Celia’s encephalopathy (PELD): new insights on fatty acids involvement, senescence and adipogenesis. PLoS ONE. 2016;11:e0158874.
Holtta-Vuori M, Salo VT, Ohsaki Y, Suster ML, Ikonen E. Alleviation of seipinopathy-related ER stress by triglyceride storage. Hum Mol Genet. 2013;22:1157–66.
Newborg JSJ, Wnek L. Inventario de desarrollo Battelle. 1996.
Araujo-Vilar D, Loidi L, Dominguez F, Cabezas-Cerrato J. Phenotypic gender differences in subjects with familial partial lipodystrophy (Dunnigan variety) due to a nuclear lamin A/C R482W mutation. Horm Metab Res. 2003;35:29–35.
Victoria B, Cabezas-Agricola JM, Gonzalez-Mendez B, et al. Reduced adipogenic gene expression in fibroblasts from a patient with type 2 congenital generalized lipodystrophy. Diabet Med. 2010;27:1178–87.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8.
Chan JL, Koda J, Heilig JS, Cochran EK, Gorden P, Oral EA, Brown RJ. Immunogenicity associated with metreleptin treatment in patients with obesity or lipodystrophy. Clin Endocrinol (Oxf). 2016;85:137–49.
Aotani D, Ebihara K, Sawamoto N, et al. Functional magnetic resonance imaging analysis of food-related brain activity in patients with lipodystrophy undergoing leptin replacement therapy. J Clin Endocrinol Metab. 2012;97:3663–71.
Paz-Filho GJ. The effects of leptin replacement on neural plasticity. Neural Plast. 2016;2016:8528934.
Farr SA, Banks WA, Morley JE. Effects of leptin on memory processing. Peptides. 2006;27:1420–5.
Fewlass DC, Noboa K, Pi-Sunyer FX, Johnston JM, Yan SD, Tezapsidis N. Obesity-related leptin regulates Alzheimer’s Abeta. FASEB J. 2004;18:1870–8.
Elmquist JK, Maratos-Flier E, Saper CB, Flier JS. Unraveling the central nervous system pathways underlying responses to leptin. Nat Neurosci. 1998;1:445–50.
Bouret SG. Neurodevelopmental actions of leptin. Brain Res. 2010;1350:2–9.
Harvey J. Leptin: a diverse regulator of neuronal function. J Neurochem. 2007;100:307–13.
Parimisetty A, Dorsemans AC, Awada R, Ravanan P, Diotel N, Lefebvre d’Hellencourt C. Secret talk between adipose tissue and central nervous system via secreted factors-an emerging frontier in the neurodegenerative research. J Neuroinflamm. 2016;13:67.
Paz-Filho G, Wong ML, Licinio J. The procognitive effects of leptin in the brain and their clinical implications. Int J Clin Pract. 2010;64:1808–12.
Russo VC, Metaxas S, Kobayashi K, Harris M, Werther GA. Antiapoptotic effects of leptin in human neuroblastoma cells. Endocrinology. 2004;145:4103–12.
Teryaeva NB. [Leptin as a neuroprotector and functional stability factor in the central neural system]. Ross Fiziol Zh Im I M Sechenova. 2013;99:1138–48.
O’Malley D, MacDonald N, Mizielinska S, Connolly CN, Irving AJ, Harvey J. Leptin promotes rapid dynamic changes in hippocampal dendritic morphology. Mol Cell Neurosci. 2007;35:559–72.
Udagawa J, Nimura M, Otani H. Leptin affects oligodendroglial development in the mouse embryonic cerebral cortex. Neuro Endocrinol Lett. 2006;27:177–82.
Procaccini C, Santopaolo M, Faicchia D, et al. Role of metabolism in neurodegenerative disorders. Metabolism. 2016;65:1376–90.
Lieb W, Beiser AS, Vasan RS, et al. Association of plasma leptin levels with incident Alzheimer disease and MRI measures of brain aging. JAMA. 2009;302:2565–72.
Bonda DJ, Stone JG, Torres SL, et al. Dysregulation of leptin signaling in Alzheimer disease: evidence for neuronal leptin resistance. J Neurochem. 2014;128:162–72.
Paz-Filho GJ, Babikian T, Asarnow R, et al. Leptin replacement improves cognitive development. PLoS ONE. 2008;3:e3098.
Hadley KB, Ryan AS, Nelson EB, Salem N. An assessment of dietary docosahexaenoic acid requirements for brain accretion and turnover during early childhood. World Rev Nutr Diet. 2009;99:97–104.
Innis SM. Dietary omega 3 fatty acids and the developing brain. Brain Res. 2008;1237:35–43.
Joffre C, Nadjar A, Lebbadi M, Calon F, Laye S. n-3 LCPUFA improves cognition: the young, the old and the sick. Prostaglandins Leukot Essent Fat Acids. 2014;91:1–20.
Kuratko CN, Barrett EC, Nelson EB, Salem N Jr. The relationship of docosahexaenoic acid (DHA) with learning and behavior in healthy children: a review. Nutrients. 2013;5:2777–810.
Bazan NG, Molina MF, Gordon WC. Docosahexaenoic acid signalolipidomics in nutrition: significance in aging, neuroinflammation, macular degeneration, Alzheimer’s, and other neurodegenerative diseases. Annu Rev Nutr. 2011;31:321–51.
Freund-Levi Y, Eriksdotter-Jonhagen M, Cederholm T, et al. Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: a randomized double-blind trial. Arch Neurol. 2006;63:1402–8.
Schaefer EJ, Bongard V, Beiser AS, et al. Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and Alzheimer disease: the Framingham Heart Study. Arch Neurol. 2006;63:1545–50.
Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS. Fish consumption and cognitive decline with age in a large community study. Arch Neurol. 2005;62:1849–53.
Rapoport SI, Rao J, Igarashi M. Brain metabolism of nutritionally essential polyunsaturated fatty acids depends on both the diet and the liver. Prostaglandins Leukot Essent Fat Acids. 2007;77:251–61.
Bazinet RP, Laye S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat Rev Neurosci. 2014;15:771–85.
Quinn JF, Raman R, Thomas RG, et al. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: a randomized trial. JAMA. 2010;304:1903–11.
Casanas-Sanchez V, Perez JA, Fabelo N, Quinto-Alemany D, Diaz ML. Docosahexaenoic (DHA) modulates phospholipid-hydroperoxide glutathione peroxidase (Gpx4) gene expression to ensure self-protection from oxidative damage in hippocampal cells. Front Physiol. 2015;6:203.
Calderon F, Kim HY. Docosahexaenoic acid promotes neurite growth in hippocampal neurons. J Neurochem. 2004;90:979–88.
Moriguchi T, Harauma A, Salem N Jr. Plasticity of mouse brain docosahexaenoic acid: modulation by diet and age. Lipids. 2013;48:343–55.
Lei E, Vacy K, Boon WC. Fatty acids and their therapeutic potential in neurological disorders. Neurochem Int. 2016;95:75–84.
Novak EM, Dyer RA, Innis SM. High dietary omega-6 fatty acids contribute to reduced docosahexaenoic acid in the developing brain and inhibit secondary neurite growth. Brain Res. 2008;1237:136–45.
van Elst K, Bruining H, Birtoli B, Terreaux C, Buitelaar JK, Kas MJ. Food for thought: dietary changes in essential fatty acid ratios and the increase in autism spectrum disorders. Neurosci Biobehav Rev. 2014;45:369–78.
Wijendran V, Downs I, Srigley CT, et al. Dietary arachidonic acid and docosahexaenoic acid regulate liver fatty acid desaturase (FADS) alternative transcript expression in suckling piglets. Prostaglandins Leukot Essent Fat Acids. 2013;89:345–50.
Acknowledgements
We thank the patient and her parents for their cooperation and collaboration in this study. This work was supported by the Fundación Mutua Madrileña and by the Instituto de Salud Carlos III and the European Regional Development Fund, FEDER (PI13/00314). SR-G was awarded a Research Fellowship by the Asociación Española de Familiares y Afectados de Lipodistrofias (AELIP). We thank Bristol-Myers-Squibb, AstraZeneca, and Aegerion Pharmaceuticals for providing metreleptin, Dr Jesús R Requena for the kind gift of SH-SY5Y cells, and David Araujo-Gonzalez for technical support in Tube-Video presentation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
DA-V received an honorarium as an expert advisor from Aegerion Pharmaceuticals; the remaining authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Araújo-Vilar, D., Domingo-Jiménez, R., Ruibal, Á. et al. Association of metreleptin treatment and dietary intervention with neurological outcomes in Celia’s encephalopathy. Eur J Hum Genet 26, 396–406 (2018). https://doi.org/10.1038/s41431-017-0052-8
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41431-017-0052-8
This article is cited by
-
Metreleptin for the treatment of progressive encephalopathy with/without lipodystrophy (PELD) in a child with progressive myoclonic epilepsy: a case report
Italian Journal of Pediatrics (2020)
-
Celia’s encephalopathy and c.974dupG in BSCL2 gene: a hidden change in a known variant
neurogenetics (2019)