Abstract
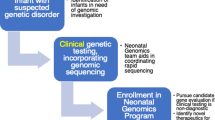
We investigated the attitudes of intensive care physicians and genetics professionals towards rapid genomic testing in neonatal and paediatric intensive care units (NICU/PICU). A mixed-methods study (surveys and interviews) was conducted at 13 Australian hospitals and three laboratories involved in multi-center implementation of rapid genomic testing. We investigated experience and confidence with genomic tests among intensivists; perceived usefulness of genomic diagnostic results; preferences for service delivery models; and implementation readiness among genetic services. The overall survey response rate was 59%, 47% for intensivists (80/170), and 75% (91/121) for genetics professionals. Intensivists reported moderate confidence with microarray tests and lower confidence with genomic tests. The majority of intensivists (77%), clinical geneticists (87%) and genetic counsellors (82%) favoured a clinical genetics-led service delivery model of genomic testing. Perceived clinical utility of genomic results was lower in the intensivist group compared to the genetics professionals group (20 v 50%, p < 0.001). Interviews (n = 6 intensivists; n = 11 genetic counselors) demonstrated support for implementation, with concerns relating to implementation environment and organizational readiness. Overall, our findings support initial implementation of genomic testing in NICU/PICU as part of an interdisciplinary service delivery model that promotes gradual adoption of genomics by the intensive care workforce while ensuring safety, sustainability, and efficiency.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Kingsmore SF, Petrikin J, Willig LK, Guest E. Emergency medical genomes: a breakthrough application of precision medicine. Genome Med. 2015;7:82.
Daoud H, Luco SM, Li R, Bareker E, Beaulieu C, Jarinova O, et al. Next-generation sequencing for diagnosis of rare diseases in the neonatal intensive care unit. CMAJ. 2016;188:E254–260.
Meng L, Pammi M, Saronwala A, Magoulas P, Ghazi AR, Vetrini F, et al. Use of exome sequencing for infants in intensive care units: ascertainment of severe single-gene disorders and effect on medical management. JAMA Pediatr. 2017;171:e173438.
Petrikin JE, Cakici JA, Clark MM, Willig LK, Sweeney NM, Farrow EG, et al. The NSIGHT1-randomized controlled trial: rapid whole-genome sequencing for accelerated etiologic diagnosis in critically ill infants. NPJ Genom Med. 2018;3:6.
Stark Z, Lunke S, Brett GR, Tan NB, Stapleton R, Kumble S, et al. Meeting the challenges of implementing rapid genomic testing in acute pediatric care. Genet Med. 2018;20:1554–63.
Stark Z, Tan TY, Chong B, Brett GR, Yap P, Walsh M, et al. A prospective evaluation of whole-exome sequencing as a first-tier molecular test in infants with suspected monogenic disorders. Genet Med. 2016;18:1090–6.
van Diemen CC, Kerstjens-Frederikse WS, Bergman KA, de Koning TJ, Sikkema-Raddatz B, van der Velde JK, et al. Rapid Targeted Genomics in Critically Ill Newborns. Pediatrics. 2017;140:e20162854.
Farnaes L, Hildreth A, Sweeney NM, Clarke MM, Chowdhury S, Nahas S, et al. Rapid whole-genome sequencing decreases infant morbidity and cost of hospitalization. NPJ Genom Med. 2018;3:10.
Mestek-Boukhibar L, Clement E, Jones WD, Drury S, Ocaka L, Gagunashvili A, et al. Rapid Paediatric Sequencing (RaPS): comprehensive real-life workflow for rapid diagnosis of critically ill children. J Med Genet. 2018;55:721–8.
Willig LK, Petrikin JE, Smith LD, Saunders CJ, Thiffault I, Miller NA, et al. Whole-genome sequencing for identification of Mendelian disorders in critically ill infants: a retrospective analysis of diagnostic and clinical findings. Lancet Resp Med. 2015;3:377–87.
Char DS, Lee SS, Magnus D, Cho M. Anticipating uncertainty and irrevocable decisions: provider perspectives on implementing whole-genome sequencing in critically ill children with heart disease. Genet Med. 2018;20:1455–61.
Cresswell and Plano Clark. Designing and conducting mixed methods research. SAGE Publications, Thousand Oaks, US, 2018.
Sukenik-Halevy R, Ludman MD, Ben-Shachar S, Raas-Rothschild A. The time-consuming demands of the practice of medical genetics in the era of advanced genomic testing. Genet Med. 2016;18:372–7.
National Society of Genetic Counselors. Professional Status Survey, 2016, Vol 2017. http://www.nsgc.org/p/cm/Id/fid=68.
Royal College of Physicians. Consultant Physicians Working With Patients. London: RCP, 2013.
Nisselle, A, Macciocca I, McKenzie, F, Vuong, H, Dunlop, K, McClaren, B, et al. Readiness of clinical genetic healthcare professionals to provide genomic medicine: an Australian census. J Genet Couns 2018;28:367–377.
Shea CM, Jacobs SR, Esserman DA, Bruce K, Weiner BJ. Organizational readiness for implementing change: a psychometric assessment of a new measure. Implement Sci. 2014;9:7.
Neuman WL. Social Research Methods; qualitative and quantitative approaches. Pearson Education Inc, Harlow, UK, 2006.
Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50.
Petrikin JE, Willig LK, Smith LD, Kingsmore SF. Rapid whole genome sequencing and precision neonatology. Semin Perinatol. 2015;39:623–31.
Smith LD, Willig LK, Kingsmore SF. Whole-exome sequencing and whole-genome sequencing in critically Ill neonates suspected to have single-gene disorders. Cold Spring Harb Perspect Med. 2015;6:a023168.
Friedman JM, Bombard Y, Cornel MC, Fernandez CV, Junker AK, Plon SE, et al. Genome-wide sequencing in acutely ill infants: genomic medicine’s critical application? Genet Med. 2018. https://doi.org/10.1038/s41436-018-0055-z.
Selkirk CG, Weissman SM, Anderson A, Hulick PJ. Physicians’ preparedness for integration of genomic and pharmacogenetic testing into practice within a major healthcare system. Genet Test Mol Biomark. 2013;17:219–25.
Bonter K, Desjardins C, Currier N, Pun J, Ashbury FD. Personalised medicine in Canada: a survey of adoption and practice in oncology, cardiology and family medicine. BMJ Open. 2011;1:bmjopen-2011–000110.
Johansen Taber KA, Dickinson BD. Pharmacogenomic knowledge gaps and educational resource needs among physicians in selected specialties. Pharmgenomics Pers Med. 2014;7:145–62.
Gray SW, Hicks-Courant K, Cronin A, Rollins BJ, Weeks JC. Physicians’ attitudes about multiplex tumor genomic testing. J Clin Oncol. 2014;32:1317–23.
Chow-White P, Ha D, Laskin J. Knowledge, attitudes, and values among physicians working with clinical genomics: a survey of medical oncologists. Hum Resour Health. 2017;15:42–51.
Bayefsky MJ, White A, Wakim P, Hull SC, Wasserman D, Chen S, et al. Views of American OB/GYNs on the ethics of prenatal whole-genome sequencing. Prenat Diagn. 2016;36:1250–6.
Johnson L-M, Valdez JM, Quinn EA, Sykes AD, McGee RB, Nuccio R, et al. Integrating next-generation sequencing into pediatric oncology practice: An assessment of physician confidence and understanding of clinical genomics. Cancer. 2017;123:2352–9.
Weipert CM, Ryan KA, Everett JN, Yashar BM, Chinnaiyan M, Scott Roberts J, et al. Physician experiences and understanding of genomic sequencing in oncology. J Genet Couns. 2018;27:187–96.
Sanson-Fisher RW. Diffusion of innovation theory for clinical change. Med J Aust. 2004;180:S55–S56.
Harris JN, Liljestrand P, Alexander GL, Goddard KA, Kaufmann T, Kolevska T, et al. Oncologists’ attitudes toward KRAS testing: a multisite study. Cancer Med. 2013;2:881–8.
Acknowledgements
This work was supported by the Victorian Government’s Operational Infrastructure Support Program and a grant from the Australian National Health & Medical Research Council (GNT1113531); the contents are solely the responsibility of the individual authors and do not reflect the views of the NHMRC. The authors thank all those who responded to the survey and all members of the Australian Genomics Health Alliance for their contribution to this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The Human Research Ethics Committee at The University of Melbourne (HREC 1646785) approved the study.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Stark, Z., Nisselle, A., McClaren, B. et al. Attitudes of Australian health professionals towards rapid genomic testing in neonatal and paediatric intensive care. Eur J Hum Genet 27, 1493–1501 (2019). https://doi.org/10.1038/s41431-019-0429-y
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41431-019-0429-y
This article is cited by
-
View of healthcare professionals on ultra-rapid genome sequencing and its future implementation in clinical practice for critically ill children
European Journal of Human Genetics (2025)
-
Next-generation nephrology: part 2—mainstreaming genomics in nephrology, a global perspective
Pediatric Nephrology (2025)
-
Self-assessed knowledge of genomic medicine among non-genetics physicians – results from a nationwide Swedish survey
Journal of Community Genetics (2025)
-
Rapid genomic testing in critically ill patients with genetic conditions: position statement by the Human Genetics Society of Australasia
European Journal of Human Genetics (2024)
-
Investigating genomic medicine practice and perceptions amongst Australian non-genetics physicians to inform education and implementation
npj Genomic Medicine (2023)