Abstract
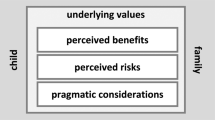
Genome sequencing (GS) studies involving healthy children can advance scientific knowledge of genetic variation. Little research has examined primary care providers’ views on using GS in this context. This study explored primary care provider perspectives on the use of GS in research and the care of healthy children. We conducted semi-structured interviews with 16 providers discussing their views on GS research and receiving results. Interviews were analyzed by thematic analysis and constant comparison. Participants were family physicians (11/16) and primary care pediatricians (5/16) in practice for >10 years (11/16). Participants valued GS in healthy children for research purposes; however, opinions diverged on using the results in primary care. Proponents valued using results for surveillance and prevention in healthy children. Skeptics questioned the clinical utility of results and the appropriateness of applying research data in primary care. Both groups shared concerns over opportunistic screening, validity, and interpretation of results, increased health system costs and inequities, and genetic discrimination. Primary care providers were ambivalent about the appropriateness and utility of GS in the care of healthy children. Providers feel unprepared and unsure of their obligations in disclosing these results. Providers do not feel they are equipped with the necessary resources and training to support their patients in using GS results in their care.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Grody WW, Thompson BH, Hudgins L. Whole-exome/genome sequencing and genomics. Pediatrics. 2013;132:211–5.
Bamshad MJ, Ng SB, Bigham AW, Tabor HK, Emond MJ, Nickerson DA, et al. Exome sequencing as a tool for Mendelian disease gene discovery. Nat Rev Genet. 2011;12:745–55.
Behati S, Tarpey PS. What is next generation sequencing? Arch Dis Child Educ Pr Ed. 2013;98:236–8.
Lionel AC, Costain G, Monfared N, Walker S, Reuter MS, Hosseini SM, et al. Improved diagnostic yield compared with targeted gene sequencing panels suggests a role for whole-genome sequencing as a first-tier genetic test. Genet Med. 2018;20:435–43.
Pareek CS, Smoczynski R, Tretyn A. Sequencing technologies and genome sequencing. J Appl Genet. 2011;52:413–5.
Bombard Y, Bach PB, Offit K. Translating genomics in cancer care. J Natl Compr Canc Netw. 2013;11:1343–53.
Golding J. The Avon Longitudinal Study of Parents and Children (ALSPAC)—study design and collaborative opportunities. Eur J Endocrinol. 2004;151:U119–23.
Boycott K, Hartley T, Adam S, Bernier F, Chong K, Fernandez BA, et al. The clinical application of genome-wide sequencing for monogenic diseases in Canada: position statement of the Canadian College of Medical Geneticists. J Med Gen. 2015;52:431–7.
Smith HS, Swint JM, Lalani SR, Yamal JM, de Oliveira Otto MC, Castellanos S, et al. Clinical application of genome and exome sequencing as a diagnostic tool for pediatric patients: a scoping review of the literature. Genet Med. 2019;21:3–16.
Parsons D, Roy A, Yang Y, Wang T, Scollon S, Bergstrom K, et al. Diagnostic yield of clinical tumor and germline whole-exome sequencing for children with solid tumor. JAMA Oncol. 2016;2:616–24.
Burke W, Antommmaria AHM, Bennett R, Botkin J, Clayton EW, Henderson GE, et al. Recommendations for returning genomic incidental findings? We need to talk! Genet Med. 2013;15:854–9.
Bombard Y, Robson M, Offit K. Revealing the incidentalome when targeting the tumor genome. J Am Med Assoc. 2013;310:795–6.
Fernandez CV, Bouffet E, Malkin D, Jabado N, O’Connell C, Avard D, et al. Attitudes of parents toward the return of targeted and incidental genomic research findings in children. Genet Med. 2014;16:633–40.
Kleiderman E, Knoppers BM, Fernandez CV, Boycott KM, Ouellette G, Wong-Rieger D, et al. Returning incidental findings from genetic research to children: views of parents of children affected by rare diseases. J Med Ethics. 2014;40:691–6.
Brothers KB, Vassy JL, Green RC. Reconciling opportunistic and population screening in clinical genomics. Mayo Clin Proc. 2019;94:103–9.
Mackley MP, Capps B. Expect the unexpected: screening for secondary findings in clinical genomics research. Brit Med Bull. 2017;122:109–22.
Sandelowski M. Sample size in qualitative research. Res Nurs Health. 1995;18:179–83.
Morse JM. Designing funded qualitative research. In: Handbook of qualitative research. CA: SAGE Publications Ltd; 1994. p. 220–35.
Strauss A, Corbin J. Basics of qualitative research: techniques and procedures for developing grounded theory. CA, USA: SAGE Publications Ltd; 1998.
Charmaz KC. Constructing grounded theory: a practical guide through qualitative analysis. London, UK: SAGE Publications Ltd; 2006.
Charmaz KC. Qualitative interviewing and grounded theory analysis. In: Holstein JA, Gubrium JF, editors. Inside interviewing: new lenses, new concerns. Thousand Oaks, CA: Sage Publications Inc; 2003. p. 311–30.
Foley SB, Rios JJ, Mgbemena VE, Robinson LS, Hampel HL, Toland AE, et al. Use of whole genome sequencing for diagnosis and discovery in the cancer genetics clinic. EBioMedicine. 2015;2:74–81.
Bennette CS, Gallego CJ, Burke W, Jarvik GP, Veenstra DL. The cost-effectiveness of returning incidental findings from next-generation genomic sequencing. Genet Med. 2015;17:587–95.
Christensen KD, Dukhovny D, Siebert U, Green RC. Assessing the costs and cost-effectiveness of genomic sequencing. J Pers Med. 2015;5:470–86.
Lemke AA, Bick D, Dimmock D, Simpson P, Veith R. Perspectives of clinical genetics professionals toward genome sequencing and incidental findings: a survey study. Clin Genet. 2013;84:230–6.
Yu J, Harrell TM, Jamal SM, Tabor HK, Bamshad MJ. Attitudes of genetics professionals towards the return of incidental results from exome and whole-genome sequencing. Am J Hum Genet. 2014;95:77–84.
Kleiderman E, Avard D, Besso A, Ali-Khan S, Sauvageau G, Hebert J. Disclosure of incidental findings in cancer genomic research: investigators’ perceptions on obligations and barriers. Clin Genet. 2015;88:320–6.
Knoppers BM, Joly Y, Jacques S, Durocher F. The emergence of an ethical duty to disclose genetic research results: international perspectives. Eur J Hum Genet. 2006;14:1170–8.
Lindor NM, Thibodeau SN, Burke W. Whole-genome sequencing in healthy. People Mayo Clin Proc. 2017;92:159–72.
Bombard Y, Miller FA, Barg CJ, Patton SJ, et al. A secondary benefit: the reproductive impact of carrier results from newborn screening for cystic fibrosis. Genet Med. 2017;19:403–11.
Bombard Y, Miller FA, Hayeems RZ, Wilson BJ, et al. Health-care providers’ views on pursuing reproductive benefit through newborn screening: the case of sickle cell disorders. Eur J Hum Genet. 2012;20:498–504.
Bombard Y, Miller A, Hayeems RZ, Avard D, Knoppers BM. Reconsidering reproductive benefit through newborn screening: a systemtic review of guidelines on preconception, prenatal and newborn screening. Eur J Hum Genet. 2010;18:751–60.
Bombard Y, Miller FA, Hayeems RZ, Avard D, Knoppers BM, Cornel MC, et al. The expansion of newborn screening: is reproductive benefit an appropriate pursuit? Nat Rev Genet. 2009;10:666–7.
Pereira S, Robinson JO, Gutierrez AM, Petersen DK, Hsu RL, Lee Ch, et al. Perceived benefits, risks and utility of newborn genomic sequencing in the BabySeq project. Pediatrics. 2019;143:S6–S13.
Green RC, Berg JS, Grody WW, Kalia SS, Korf BR, Martin CL, et al. ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genet Med. 2013;15:565–74.
Ormondroyd E, Mackley MP, Blair E, Craft J, Knight JC, Taylor JC, et al. “Not pathogenic until proven otherwise”: perspectives of UK clinical genomics professionals toward secondary findings in context of a Genomic Medicine Multidisiplinary Team and the 100,000 genomes project. Genet Med. 2018;20:320–8.
Vassy JL, Christensen KD, Schonman EF, Blout CL, Robinson JO, Krier JB, et al. The impact of whole-genome sequencing on the primary care and outcomes of healthy adult patients: a pilot randomized trial. Ann Intern Med. 2017;167:159–69.
Delanne J, Nambot S, Chassagne A, Putois O, Pelissier A, Peyron C, et al. Secondary findings from whole-exome/genome sequencing evaluating stakeholder perspectives. A review of the literature. Eur J Med Genet. 2019;62:103529.
Strong KA, Zusevics KL, Bick D, Veith R. Views of primary care providers regarding the return of genome sequencing incidental findings. Clin Genet. 2014;8:461–8.
Acknowledgements
We would like to thank the primary care providers that participated in our study for sharing their valuable time and insights with us.
Funding
The research presented in this paper was supported by the University of Toronto’s McLaughlin Centre. YB was supported by a New Investigator Award from the Canadian Institutes of Health Research (CIHR) during this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Joshi, E., Mighton, C., Clausen, M. et al. Primary care provider perspectives on using genomic sequencing in the care of healthy children. Eur J Hum Genet 28, 551–557 (2020). https://doi.org/10.1038/s41431-019-0547-6
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41431-019-0547-6