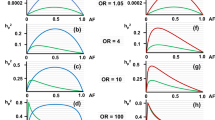
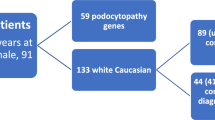
Abstract
Hypophosphatasia (HPP) is caused by pathogenic variants in the ALPL gene. There is a large continuum in the severity, ranging from a lethal perinatal form to dental issues. We analyzed a cohort of 424 HPP patients from European geographic origin or ancestry. Using 3D modeling and results of functional tests we classified ALPL pathogenic variants according to their dominant negative effect (DNE) and their severity. The cohort was described by the genotypes resulting from alleles s (severe recessive), Sd (severe dominant), and m (moderate). Many recurrent variants showed a regional anchor pointing out founder effects rather than multiple mutational events. Homozygosity was an aggravating factor of the severity and moderate alleles were rare both in number and frequency. Pathogenic variants with DNE were found in both recessive and dominant HPP. Sixty percent of the adults tested were heterozygous for a variant showing no DNE, suggesting another mechanism of dominance like haploinsufficiency. Adults with dominant HPP without DNE were found statistically less severely affected than adults with DNE variants. Adults with dominant HPP without DNE represent a new clinical entity mostly diagnosed from 2010s, characterized by nonspecific signs of HPP and low alkaline phosphatase, and for which a high prevalence is expected. In conclusion, the genetic composition of our cohort suggests a nosology with 3 clinical forms: severe HPP is recessive and rare, moderate HPP is recessive or dominant and more common, and mild HPP, characterized by low alkaline phosphatase and unspecific clinical signs, is dominantly inherited and very common.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The authors state the availability of data and materials for replication of findings.
References
Mornet E. Hypophosphatasia. Orphanet J Rare Dis. 2007;2:40.
Mornet E, Nunes ME. Hypophosphatasia. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, et al., editors. GeneReviews((R)). Seattle (WA); University of Washington, 1993.
Whyte MP. Hypophosphatasia: an overview For 2017. Bone. 2017;102:15–25.
Mornet E, Yvard A, Taillandier A, Fauvert D, Simon-Bouy B. A molecular-based estimation of the prevalence of hypophosphatasia in the European population. Ann Hum Genet. 2011;75:439–45.
Fraser D. Hypophosphatasia. Am J Med. 1957;22:730–46.
Mornet E. Hypophosphatasia: the mutations in the tissue-nonspecific alkaline phosphatase gene. Hum Mutat. 2000;15:309–15.
Mornet E. Genetics of hypophosphatasia. Arch Pediatr. 2017;24:5S51–5S6.
Mornet E. Hypophosphatasia. Metabolism 2018;82:142–55.
Linglart A, Biosse-Duplan M. Hypophosphatasia. Curr Osteoporos Rep. 2016;14:95–105.
Whyte MP, Zhang F, Wenkert D, McAlister WH, Mack KE, Benigno MC, et al. Hypophosphatasia: validation and expansion of the clinical nosology for children from 25 years experience with 173 pediatric patients. Bone. 2015;75:229–39.
Taillandier A, Domingues C, Dufour A, Debiais F, Guggenbuhl P, Roux C, et al. Genetic analysis of adults heterozygous for ALPL mutations. J Bone Min Metab. 2018;36:723–33.
Whyte MP, Greenberg CR, Salman NJ, Bober MB, McAlister WH, Wenkert D, et al. Enzyme-replacement therapy in life-threatening hypophosphatasia. N Engl J Med. 2012;366:904–13.
Whyte MP. Atypical femoral fractures, bisphosphonates, and adult hypophosphatasia. J Bone Min Res. 2009;24:1132–4.
Sutton RA, Mumm S, Coburn SP, Ericson KL, Whyte MP. “Atypical femoral fractures” during bisphosphonate exposure in adult hypophosphatasia. J Bone Min Res. 2012;27:987–94.
Deeb AA, Bruce SN, Morris AA, Cheetham TD. Infantile hypophosphatasia: disappointing results of treatment. Acta Paediatr. 2000;89:730–3.
Baujat G, Michot C, Le Quan Sang KH, Cormier-Daire V. Perinatal and infantile hypophosphatasia: clinical features and treatment. Arch Pediatr. 2017;24:5S61–5S5.
Linglart A, Salles JP. Hypophosphatasia: the contribution of imaging. Arch Pediatr. 2017;24:5S74–5S9.
Bloch-Zupan A. Hypophosphatasia: diagnosis and clinical signs - a dental surgeon perspective. Int J Paediatr Dent. 2016;26:426–38.
Briot K, Roux C. Adult hypophosphatasia. Curr Opin Rheumatol. 2016;28:448–51.
Dahir KM, Tilden DR, Warner JL, Bastarache L, Smith DK, Gifford A, et al. Rare variants in the gene ALPL that cause hypophosphatasia are strongly associated with ovarian and uterine disorders. J Clin Endocrinol Metab. 2018;103:2234–43.
Zurutuza L, Muller F, Gibrat JF, Taillandier A, Simon-Bouy B, Serre JL, et al. Correlations of genotype and phenotype in hypophosphatasia. Hum Mol Genet. 1999;8:1039–46.
Yang H, Wang L, Geng J, Yu T, Yao RE, Shen Y, et al. Characterization of six missense mutations in the tissue-nonspecific alkaline phosphatase (TNSALP) gene in Chinese children with hypophosphatasia. Cell Physiol Biochem. 2013;32:635–44.
Takinami H, Goseki-Sone M, Watanabe H, Orimo H, Hamatani R, Fukushi-Irie M, et al. The mutant (F310L and V365I) tissue-nonspecific alkaline phosphatase gene from hypophosphatasia. J Med Dent Sci. 2004;51:67–74.
Taillandier A, Sallinen SL, Brun-Heath I, De Mazancourt P, Serre JL, Mornet E. Childhood hypophosphatasia due to a de novo missense mutation in the tissue-nonspecific alkaline phosphatase gene. J Clin Endocrinol Metab. 2005;90:2436–9.
Taillandier A, Lia-Baldini AS, Mouchard M, Robin B, Muller F, Simon-Bouy B, et al. Twelve novel mutations in the tissue-nonspecific alkaline phosphatase gene (ALPL) in patients with various forms of hypophosphatasia. Hum Mutat. 2001;18:83–4.
Lia-Baldini AS, Muller F, Taillandier A, Gibrat JF, Mouchard M, Robin B, et al. A molecular approach to dominance in hypophosphatasia. Hum Genet. 2001;109:99–108.
Fukushi-Irie M, Ito M, Amaya Y, Amizuka N, Ozawa H, Omura S, et al. Possible interference between tissue-non-specific alkaline phosphatase with an Arg54->Cys substitution and acounterpart with an Asp277->Ala substitution found in a compound heterozygote associated with severe hypophosphatasia. Biochem J. 2000;348:633–42.
Fauvert D, Brun-Heath I, Lia-Baldini AS, Bellazi L, Taillandier A, Serre JL, et al. Mild forms of hypophosphatasia mostly result from dominant negative effect of severe alleles or from compound heterozygosity for severe and moderate alleles. BMC Med Genet. 2009;10:51.
Orimo H, Girschick HJ, Goseki-Sone M, Ito M, Oda K, Shimada T. Mutational analysis and functional correlation with phenotype in German patients with childhood-type hypophosphatasia. J Bone Min Res. 2001;16:2313–9.
Satou Y, Al-Shawafi HA, Sultana S, Makita S, Sohda M, Oda K. Disulfide bonds are critical for tissue-nonspecific alkaline phosphatase function revealed by analysis of mutant proteins bearing a C(201)-Y or C(489)-S substitution associated with severe hypophosphatasia. Biochim Biophys Acta. 2012;1822:581–8.
Brun-Heath I, Taillandier A, Serre JL, Mornet E. Characterization of 11 novel mutations in the tissue non-specific alkaline phosphatase gene responsible for hypophosphatasia and genotype-phenotype correlations. Mol Genet Metab. 2005;84:273–7.
Mentrup B, Girschick H, Jakob F, Hofmann C. A homozygous intronic branch-point deletion in the ALPL gene causes infantile hypophosphatasia. Bone. 2017;94:75–83.
Spentchian M, Merrien Y, Herasse M, Dobbie Z, Glaser D, Holder SE, et al. Severe hypophosphatasia: characterization of fifteen novel mutations in the ALPL gene. Hum Mutat. 2003;22:105–6.
Del Angel G, Reynders J, Negron C, Steinbrecher T, Mornet E. Large-scale in vitro functional testing and novel variant scoring via protein modeling provide insights into alkaline phosphatase activity in hypophosphatasia. Hum Mutat. 2020;41:1250–62.
Taillandier A, Domingues C, De Cazanove C, Porquet-Bordes V, Monnot S, Kiffer-Moreira T, et al. Molecular diagnosis of hypophosphatasia and differential diagnosis by targeted Next Generation Sequencing. Mol Genet Metab. 2015;116:215–20.
Derouault P, Parfait B, Moulinas R, Barrot CC, Sturtz F, Merillou S, et al. ‘COV’COP’ allows to detect CNVs responsible for inherited diseases among amplicons sequencing data. Bioinformatics. 2017;33:1586–8.
den Dunnen JT, Dalgleish R, Maglott DR, Hart RK, Greenblatt MS, McGowan-Jordan J, et al. HGVS Recommendations for the Description of Sequence Variants: 2016 Update. Hum Mutat. 2016;37:564–9.
Mornet E, Stura E, Lia-Baldini AS, Stigbrand T, Menez A, Le DuMH. Structural evidence for a functional role of human tissue nonspecific alkaline phosphatase in bone mineralization. J Biol Chem. 2001;276:31171–8.
Zhang H, Ke YH, Wang C, Yue H, Hu WW, Gu JM, et al. Identification of the mutations in the tissue-nonspecific alkaline phosphatase gene in two Chinese families with hypophosphatasia. Arch Med Res. 2012;43:21–30.
Herasse M, Spentchian M, Taillandier A, Mornet E. Evidence of a founder effect for the tissue-nonspecific alkaline phosphatase (TNSALP) gene E174K mutation in hypophosphatasia patients. Eur J Hum Genet. 2002;10:666–8.
Orton NC, Innes AM, Chudley AE, Bech-Hansen NT. Unique disease heritage of the Dutch-German Mennonite population. Am J Med Genet A. 2008;146A:1072–87.
Wenkert D, McAlister WH, Coburn SP, Zerega JA, Ryan LM, Ericson KL, et al. Hypophosphatasia: nonlethal disease despite skeletal presentation in utero (17 new cases and literature review). J Bone Min Res. 2011;26:2389–98.
Riancho-Zarrabeitia L, Garcia-Unzueta M, Tenorio JA, Gomez-Gerique JA, Ruiz Perez VL, Heath KE, et al. Clinical, biochemical and genetic spectrum of low alkaline phosphatase levels in adults. Eur J Intern Med. 2016;29:40–5.
Mornet E. Molecular Genetics of Hypophosphatasia and Phenotype-Genotype Correlations. Subcell Biochem. 2015;76:25–43.
Tenorio J, Alvarez I, Riancho-Zarrabeitia L, Martos-Moreno GA, Mandrile G, de la Flor Crespo M, et al. Molecular and clinical analysis of ALPL in a cohort of patients with suspicion of Hypophosphatasia. Am J Med Genet A. 2017;173:601–10.
Avigad S, Kleiman S, Weinstein M, Cohen BE, Schwartz G, Woo SL, et al. Compound heterozygosity in nonphenylketonuria hyperphenylalanemia: the contribution of mutations for classical phenylketonuria. Am J Hum Genet. 1991;49:393–9.
Allen KJ, Gurrin LC, Constantine CC, Osborne NJ, Delatycki MB, Nicoll AJ, et al. Iron-overload-related disease in HFE hereditary hemochromatosis. N Engl J Med. 2008;358:221–30.
Ohno K, Suzuki K. Multiple abnormal beta-hexosaminidase alpha chain mRNAs in a compound-heterozygous Ashkenazi Jewish patient with Tay-Sachs disease. J Biol Chem. 1988;263:18563–7.
Mornet E, Crete P, Kuttenn F, Raux-Demay MC, Boue J, White PC, et al. Distribution of deletions and seven point mutations on CYP21B genes in three clinical forms of steroid 21-hydroxylase deficiency. Am J Hum Genet. 1991;48:79–88.
Komaru K, Ishida-Okumura Y, Numa-Kinjoh N, Hasegawa T, Oda K. Molecular and cellular basis of hypophosphatasia. J Oral Biosci. 2019;61:141–8.
Acknowledgements
The authors would like to thank the clinicians from 32 countries who gave us the opportunity to test their patients.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
EM received honoraria from Alexion Pharmaceutical for expertize and presentations.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Mornet, E., Taillandier, A., Domingues, C. et al. Hypophosphatasia: a genetic-based nosology and new insights in genotype-phenotype correlation. Eur J Hum Genet 29, 289–299 (2021). https://doi.org/10.1038/s41431-020-00732-6
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41431-020-00732-6
This article is cited by
-
AI-assisted phenotyping in a zebrafish hypophosphatasia model enables early and precise detection of skeletal alterations
Scientific Reports (2025)
-
Diagnosis and Treatment of Hypophosphatasia
Calcified Tissue International (2025)
-
Medical Management of Hypophosphatasia: Review of Data on Asfotase Alfa
Current Osteoporosis Reports (2025)
-
Pathophysiology of Femoral Fractures in Hypophosphatasia
Current Osteoporosis Reports (2025)
-
Adult-onset hypophosphatasia diagnosed after consecutive tooth loss during orthodontic treatment: a case report
Journal of Medical Case Reports (2024)