Abstract
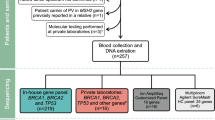
When hereditary breast and ovarian cancer (HBOC) due to a BRCA1/BRCA2 germline pathogenic variant is diagnosed, the proband will be asked to inform other at-risk family members. In the Netherlands, a guideline was introduced in 2012 which provided detailed recommendations regarding this proband-mediated procedure. We now evaluated the uptake of predictive BRCA1/BRCA2 testing in 40 consecutive HBOC families diagnosed in our centre in 2014. We performed a retrospective observational study of all 40 families in which a pathogenic BRCA1/BRCA2 germline variant was identified during 2014. We scored the uptake of predictive and confirmatory testing by the end of 2018 and explored factors associated with the level of uptake. Two families were excluded. In the remaining 38 families, among 239 family members ≥18 years at 50% risk of being a mutation carrier or at 25% risk if the family member at 50% risk was deceased, 102 (43%) were tested. Among 108 females 25–75 years of age at 50% risk, 59 (55%) underwent predictive or confirmatory testing, and among 43 males at 50% risk with daughters ≥18 years, 22 (51%) were tested. Factors which complicated cascade screening included family members living abroad, probands not wanting to share information and limited pedigree information. In conclusion, the standard proband-mediated procedure of informing relatives seems to be far from optimal. We suggest a tailored approach for each family, including the option of a direct approach to at-risk family members by the geneticist. In addition, we suggest detailed monitoring and follow-up of families.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Szabo CI, King MC. Inherited breast and ovarian cancer. Hum Mol Genet. 1995;4:1811–7.
Hereditary Tumours. Guidelines for Diagnosis and Prevention. Leiden:The Netherlands Foundation for the Detection of Hereditary Tumours and Dutch Society for Clinical Genetics; 2010. ISBN 978-90-806183-2-9 (in Dutch).
Golmard L, Delnatte C, Laugé A, Moncoutier V, Lefol C, Abidallah K, et al. Breast and ovarian cancer predisposition due to de novo BRCA1 and BRCA2 mutations. Oncogene. 2016;35:1324–7.
Tuffaha HW, Mitchell A, Ward BL, Connely L, Butler JRG, Norris S, et al. Cost-effectiveness analysis of germ-line BRCA testing in women with breast cancer and cascade testing in family members of mutation carriers. Genet Med. 2018;20:985–94.
Menko FH, ter Stege JA, van der Kolk LE, Jeanson KN, Schats W, Moha DA, et al. The uptake of presymptomatic genetic testing in hereditary breast-ovarian cancer and Lynch syndrome: a systematic review of the literature and implications for clinical practice. Fam Cancer. 2019;18:127–35.
Parker M, Lucassen A. Using a genetic test result in the care of family members: how does the duty to confidentiality apply? Eur J Hum Genet. 2018;26:955–9.
Derbez B, de Pauw A, Stoppa-Lyonnet D, de Montgolfier S. Supporting disclosure of genetic information to family members: professional practice and timelines in cancer genetics. Fam Cancer. 2017;16:447–57.
D’Audiffret van Haecke D, de Montgolfier S. Genetic diseases and information to relatives: practical and ethical issues for professionals after introduction of a legal framework in France. Eur J Hum Genet. 2018;26:786–95.
Seppälä TT, Pylvänäinen K, Mecklin J-P. Uptake of genetic testing by the children of Lynch syndrome variant carriers across three generations. Eur J Hum Genet. 2017;25:1237–45.
Nicolaidis C, Ming C, Pedrazzani C, van der Horst T, Kaiser-Grolimund A, Ademi Z, et al. Challenges and opportunities for cancer predisposition cascade screening for hereditary breast and ovarian cancer and Lynch syndrome in Switzerland: findings from an international workshop. Public Health Genom. 2018;21:121–32.
Menko FH, Aalfs, Henneman L, Stol Y, Wijdenes M, Otten E, et al. Dutch society for clinical genetics. Informing family members of individuals with Lynch syndrome: a guideline for clinical geneticists. Fam Cancer. 2013;12:319–24.
Sanz J, Ramón y Cajal T, Torres A, Darder E, Gadea N, Velasco A, et al. Uptake of predictive testing among relatives of BRCA1 and BRCA2 families: a multicenter study in northeastern Spain. Fam Cancer. 2010;9:297–304.
Sermijn E, Delesie L, Deschepper E, Pauwels I, Bonduelle M, Teugels E, et al. The impact of an interventional counseling procedure in families with a BRCA1/2 gene mutation: efficacy and safety. Fam Cancer. 2016;15:155–62.
Suthers GK, Armstrong J, McCormack J, Trott D. Letting the family know: balancing ethics and effectiveness when notifying relatives about genetic testing for a familial disorder. J Med Genet. 2006;43:665–70.
Daly MB, Montgomery S, Bingler R, Ruth K. Communicating genetic test results within the family: is it lost in translation? A survey of relatives in the randomized six-step study. Fam Cancer. 2016;15:697–706.
Brooks L, Lennard F, Shenton A, Lalloo F, Ambus I, Ardern-Jones A, et al. BRCA1/2 predictive testing: a study of uptake in two centres. Eur J Hum Genet. 2004;12:654–62.
Holloway SM, Bernhard B, Campbell H, Lam WWK. Uptake of testing for BRCA1/2 mutations in South East Scotland. Eur J Hum Genet. 2007;16:906–12.
Tung N, Domchek SM, Stadler Z, Nathanson KL, Couch F, Garber JE, et al. Counselling framework for moderate-penetrance cancer-susceptibility mutations. Nat Rev Clin Oncol. 2016;13:581–8.
ACOG Committee Opinion. Cascade testing: testing women for known hereditary genetic mutations associated with cancer. Obstet Gynaecol. 2018;131:e31–4.
Jacobs C, Patch C, Michie S. Communication about genetic testing with breast and ovarian cancer patients: a scoping review. Eur J Hum Genet. 2019;27:511–24.
de Geus E, Eijzenga W, Menko FH, Sijmons RH, de Haes CJM, Aalfs CM, et al. Design and feasibility of an intervention to support cancer genetic counselees in informing their at-risk relatives. J Genet Couns. 2016;25:1179–87.
Claes E, Evers-Kiebooms G, Boogaerts A, Decruyenaere M, Denayer L, Legius E. Communication with close and distant relatives in the context of genetic testing for hereditary breast and ovarian cancer in cancer patients. Am J Med Genet A. 2003;116A:11–9.
Katapodi MC, Viassolo V, Caiata-Zufferey M, Nikolaidis K, Bührer-Landolt R, Buerki R, et al. Cancer predisposition cascade screening for hereditary breast/ ovarian cancer and Lynch syndromes in Switzerland: study protocol. JMIR Res Protoc. 2017;6:e184.
Dove ES, Chico V, Fay M, Laurie G, Lucassen AM, Postan E. Familial genetic risk: how can we better navigate patient confidentiality and appropriate risk disclosure to relatives? J Med Ethics. 2019;45:504–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Menko, F.H., Jeanson, K.N., Bleiker, E.M.A. et al. The uptake of predictive DNA testing in 40 families with a pathogenic BRCA1/BRCA2 variant. An evaluation of the proband-mediated procedure. Eur J Hum Genet 28, 1020–1027 (2020). https://doi.org/10.1038/s41431-020-0618-8
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41431-020-0618-8
This article is cited by
-
Strategies to improve implementation of cascade testing in hereditary cancer syndromes: a systematic review
npj Genomic Medicine (2024)
-
Clinician perspectives on policy approaches to genetic risk disclosure in families
Familial Cancer (2024)
-
A tailored approach to informing relatives at risk of inherited cardiac conditions: results of a randomised controlled trial
European Journal of Human Genetics (2022)
-
30 year experience of index case identification and outcomes of cascade testing in high-risk breast and colorectal cancer predisposition genes
European Journal of Human Genetics (2022)
-
A genetic researcher’s devil’s dilemma: Warn relatives about their genetic risk or respect confidentiality agreements with research participants?
BMC Medical Ethics (2021)