Abstract
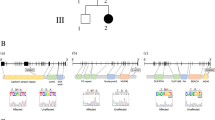
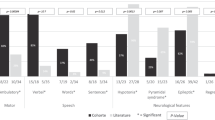
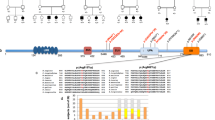
Cause of complex dyskinesia remains elusive in some patients. A homozygous missense variant leading to drastic decrease of PDE2A enzymatic activity was reported in one patient with childhood-onset choreodystonia preceded by paroxysmal dyskinesia and associated with cognitive impairment and interictal EEG abnormalities. Here, we report three new cases with biallelic PDE2A variants identified by trio whole-exome sequencing. Mitochondria network was analyzed after Mitotracker™ Red staining in control and mutated primary fibroblasts. Analysis of retrospective video of patients’ movement disorder and refinement of phenotype was carried out. We identified a homozygous gain of stop codon variant c.1180C>T; p.(Gln394*) in PDE2A in siblings and compound heterozygous variants in young adult: a missense c.446C>T; p.(Pro149Leu) and splice-site variant c.1922+5G>A predicted and shown to produce an out of frame transcript lacking exon 22. All three patients had cognitive impairment or developmental delay. The phenotype of the two oldest patients, aged 9 and 26, was characterized by childhood-onset refractory paroxysmal dyskinesia initially misdiagnosed as epilepsy due to interictal EEG abnormalities. The youngest patient showed a proven epilepsy at the age of 4 months and no paroxysmal dyskinesia at 15 months. Interestingly, analysis of the fibroblasts with the biallelic variants in PDE2A variants revealed mitochondria network morphology changes. Together with previously reported case, our three patients confirm that biallelic PDE2A variants are a cause of childhood-onset refractory paroxysmal dyskinesia with cognitive impairment, sometimes associated with choreodystonia and interictal baseline EEG abnormalities or epilepsy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bhatia KP. Paroxysmal dyskinesias. Mov Disord. 2011;26:1157–65.
Gardiner AR, Jaffer F, Dale RC, Labrum R, Erro R, Meyer E, et al. The clinical and genetic heterogeneity of paroxysmal dyskinesias. Brain. 2015;138:3567–80.
McGuire S, Chanchani S, Khurana DS. Paroxysmal dyskinesias. Semin Pediatr Neurol. 2018;25:75–81.
Ebrahimi-Fakhari D, Saffari A, Westenberger A, Klein C. The evolving spectrum of PRRT2-associated paroxysmal diseases. Brain. 2015;138:3476–95.
Schneider SA, Paisan-Ruiz C, Garcia-Gorostiaga I, Quinn NP, Weber YC, Lerche H, et al. GLUT1 gene mutations cause sporadic paroxysmal exercise-induced dyskinesias. Mov Disord. 2009;24:1684–8.
Erro R, Bhatia KP, Espay AJ, Striano P. The epileptic and nonepileptic spectrum of paroxysmal dyskinesias: channelopathies, synaptopathies, and transportopathies: the pathophysiology of paroxysmal dyskinesias. Mov Disord. 2017;32:310–8.
Chen D-H, Méneret A, Friedman JR, Korvatscha O, Gad A, Bonkowski ES, et al. ADCY5-related dyskinesia: broader spectrum and genotype–phenotype correlations. Neurology. 2015;85:2026–35.
Salpietro V, Perez-Dueñas B, Nakashima K, San Antonio-Arce V, Manole A, Efthymiou S, et al. A homozygous loss-of-function mutation in PDE2A associated to early-onset hereditary chorea: a homozygous PDE2A mutation causing chorea. Mov Disord. 2018;33:482–8.
Monterisi S, Lobo MJ, Livie C, Castle C, Weinberger M, Baillie G et al. PDE2A2 regulates mitochondria morphology and apoptotic cell death via local modulation of cAMP/PKA signalling. eLife. 2017;6:1–20.
Roth S, Heintzmann R. Optical photon reassignment with increased axial resolution by structured illumination. Methods Appl Fluoresc. 2016;4:045005.
Kirk EP, Barlow-Stewart K, Selvanathan A, Josephi-Taylor S, Worgan L, Rajagopalan S, et al. Beyond the panel: preconception screening in consanguineous couples using the TruSight One “clinical exome”. Genet Med. 2018. https://doi.org/10.1038/s41436-018-0082-9.
Souirti Z, Landré E, Mellerio C, Devaux B, Chassoux F. Neural network underlying ictal pouting (“chapeau de gendarme”) in frontal lobe epilepsy. Epilepsy Behav. 2014;37:249–57.
Exome Aggregation Consortium, Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–91.
Coubes P, Roubertie A, Vayssiere N, Hemm S, Echenne B. Treatment of DYT1-generalised dystonia by stimulation of the internal globus pallidus. Lancet. 2000;355:2220–1.
van Coller R, Slabbert P, Vaidyanathan J, Schutte C. Successful treatment of disabling paroxysmal nonkinesigenic dyskinesia with deep brain stimulation of the globus pallidus internus. Stereotact Funct Neurosurg. 2014;92:388–92.
Candela S, Vanegas MI, Darling A, Ortigoza-Escobar JD, Alamar M, Muchart J, et al. Frameless robot-assisted pallidal deep brain stimulation surgery in pediatric patients with movement disorders: precision and short-term clinical results. J Neurosurg Pediatr. 2018;22:416–25.
Narayanan DL, Deshpande D, Das Bhowmik A, Varma DR, Dalal A. Familial choreoathetosis due to novel heterozygous mutation in PDE10A. Am J Med Genet A. 2018;176:146–50.
Carecchio M, Mencacci NE. Emerging monogenic complex hyperkinetic disorders. Curr Neurol Neurosci Rep. 2017;17:97–107. https://doi.org/10.1007/s11910-017-0806-2.
Niccolini F, Mencacci NE, Yousaf T, Rabiner EA, Salpietro V, Pagano G et al. PDE10A and ADCY5 mutations linked to molecular and microstructural basal ganglia pathology: PDE10A and ADCY5 Mutations Pathology. Mov Disord. 2018;33:1961–65. https://doi.org/10.1002/mds.27523.
Conti M, Beavo J. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem. 2007;76:481–511.
Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev. 2006;58:488–520.
Bingham J, Sudarsanam S, Srinivasan S. Profiling human phosphodiesterase genes and splice isoforms. Biochem Biophys Res Commun. 2006;350:25–32.
Bender AT, Beavo JA. Specific localized expression of cGMP PDEs in Purkinje neurons and macrophages. Neurochem Int. 2004;45:853–7.
Shelly M, Lim BK, Cancedda L, Heilshorn SC, Gao H, Poor MM. Local and long-range reciprocal regulation of cAMP and cGMP in axon/dendrite formation. Science. 2010;327:547–52.
Shen K, Cowan CW. Guidance molecules in synapse formation and plasticity. Cold Spring Harb Perspect Biol. 2010;2:a001842.
Averaimo S, Nicol X. Intermingled cAMP, cGMP and calcium spatiotemporal dynamics in developing neuronal circuits. Front Cell Neurosci. 2014;8:1–10. https://doi.org/10.3389/fncel.2014.00376.
Akiyama H, Fukuda T, Tojima T, Nikolaev VO, Kamiguchi H. Cyclic nucleotide control of microtubule dynamics for axon guidance. J Neurosci. 2016;36:5636–49.
Diggle CP, Sukoff Rizzo SJ, Popiolek M, Hinttala R, Schülke JP, Kurian MA, et al. Biallelic mutations in PDE10A lead to loss of striatal PDE10A and a hyperkinetic movement disorder with onset in infancy. Am J Hum Genet. 2016;98:735–43.
Mencacci NE, Kamsteeg E-J, Nakashima K, R’Bibo L, Lynch DS, Balint B, et al. De Novo mutations in PDE10A cause childhood-onset chorea with bilateral striatal lesions. Am J Hum Genet. 2016;98:763–71.
Chang FCF, Westenberger A, Dale RC, Smith M, Pall HS, Perez-Duenas B, et al. Phenotypic insights into ADCY5-associated disease. Mov Disord J Mov Disord Soc. 2016;31:1033–40.
Friedman JR, Méneret A, Chen D-H, Trouillard O, Vidailhet M, Raskind WH, et al. ADCY5 mutation carriers display pleiotropic paroxysmal day and nighttime dyskinesias. Mov Disord J Mov Disord Soc. 2016;31:147–8.
Acin-Perez R, Russwurm M, Günnewig K, Gertz M, Zoidl G, Ramos L, et al. A phosphodiesterase 2A isoform localized to mitochondria regulates respiration. J Biol Chem. 2011;286:30423–32.
Smirnova E, Shurland DL, Ryazantsev SN, van der Bliek AM. A human dynamin-related protein controls the distribution of mitochondria. J Cell Biol. 1998;143:351–8.
Huang P, Yu T, Yoon Y. Mitochondrial clustering induced by overexpression of the mitochondrial fusion protein Mfn2 causes mitochondrial dysfunction and cell death. Eur J Cell Biol. 2007;86:289–302.
Acknowledgements
We would like to thank the patients and their parents for their participation in this study. This work was funded by CNRS, INSERM, Université de Strasbourg.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Doummar, D., Dentel, C., Lyautey, R. et al. Biallelic PDE2A variants: a new cause of syndromic paroxysmal dyskinesia. Eur J Hum Genet 28, 1403–1413 (2020). https://doi.org/10.1038/s41431-020-0641-9
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41431-020-0641-9
This article is cited by
-
Longevity, enhanced memory, and altered density of dendritic spines in hippocampal CA3 and dentate gyrus after hemizygous deletion of Pde2a in mice
Neuropsychopharmacology (2025)
-
Modulation of cAMP/cGMP signaling as prevention of congenital heart defects in Pde2A deficient embryos: a matter of oxidative stress
Cell Death & Disease (2024)
-
Whole-genome methylation analysis reveals epigenetic variation between wild-type and nontransgenic cloned, ASMT transgenic cloned dairy goats generated by the somatic cell nuclear transfer
Journal of Animal Science and Biotechnology (2022)
-
Systematic elucidation of the pharmacological mechanisms of Rhynchophylline for treating epilepsy via network pharmacology
BMC Complementary Medicine and Therapies (2021)
-
Emerging and converging molecular mechanisms in dystonia
Journal of Neural Transmission (2021)