Abstract
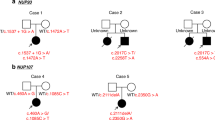
Nail-patella syndrome (NPS) is a multi-system disorder characterized by hypoplastic nails, hypoplastic patella, skeletal deformities, and iliac horns, which is caused by heterozygous variants of LMX1B. Nephropathy ranging from mild urinary abnormality to end-stage renal disease occurs in some individuals with NPS. Because of the low prevalence of NPS and the lack of longitudinal studies of its kidney involvement, the clinical, pathological, and genetic features characterizing severe nephropathy remain unclear. We conducted a Japanese survey of NPS with nephropathy, and analyzed their clinical course, pathological features, and factors associated with severe renal phenotype. LMX1B gene analysis and luciferase reporter assay were also performed. Among 13 NPS nephropathy cases with genetic validation, 5 patients who had moderate-to-massive proteinuria progressed to advanced chronic kidney disease or end-stage renal disease. Pathological findings in the early phase did not necessarily correlate with renal prognosis. Variants associated with deteriorated renal function including a novel variants were confined to the N-terminal region of the LIM domain and a short sequence in the LMX1B homeodomain, which were distinct from reported variants found in isolated nephropathy without extrarenal manifestation (LMX1B-associated nephropathy). Luciferase reporter analysis demonstrated that variants in patients with severe renal phenotype caused haploinsufficiency, but no dominant-negative effects on promoter activation. A distinct proportion of NPS nephropathy patients progressed to end-stage renal disease in adolescence or young adulthood. Patients with moderate or severe proteinuria, especially those with variants in specific regions of LMX1B, should be monitored for potential deterioration of renal function.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Mino RA, Mino VH, Livingstone RG. Osseous dysplasia and dystrophy of the nails; review of the literature and report of a case. Am J Roentgenol Radium Ther. 1948;60:633–41.
Dreyer SD, Zhou G, Baldini A, Winterpacht A, Zabel B, Cole W, et al. Mutations in LMX1B cause abnormal skeletal patterning and renal dysplasia in nail patella syndrome. Nat Genet. 1998;19:47–50.
Hawkins CF, Smith OE. Renal dysplasia in a family with multiple hereditary abnormalities including iliac horns. Lancet. 1950;1:803–8.
Looij BJ, te Slaa RL, Hogewind BL, van de Kamp JJ. Genetic counselling in hereditary osteo-onychodysplasia (HOOD, nail-patella syndrome) with nephropathy. J Med Genet. 1988;25:682–6.
Lemley KV. Kidney disease in nail-patella syndrome. Pediatr Nephrol. 2009;24:2345–54.
Sweeney E, Fryer A, Mountford R, Green A, McIntosh I. Nail patella syndrome: a review of the phenotype aided by developmental biology. J Med Genet. 2003;40:153–62.
Bongers EM, Huysmans FT, Levtchenko E, de Rooy JW, Blickman JG, Admiraal RJ, et al. Genotype-phenotype studies in nail-patella syndrome show that LMX1B mutation location is involved in the risk of developing nephropathy. Eur J Hum Genet. 2005;13:935–46.
Hoyer JR, Michael AF, Vernier RL. Renal disease in nail-patella syndrome: clinical and morphologic studies. Kidney Int. 1972;2:231–8.
Heidet L, Bongers EM, Sich M, Zhang SY, Loirat C, Meyrier A, et al. In vivo expression of putative LMX1B targets in nail-patella syndrome kidneys. Am J Pathol. 2003;163:145–55.
Clough MV, Hamlington JD, McIntosh I. Restricted distribution of loss-of-function mutations within the LMX1B genes of nail-patella syndrome patients. Hum Mutat. 1999;14:459–65.
Harita Y, Kitanaka S, Isojima T, Ashida A, Hattori M. Spectrum of LMX1B mutations: from nail-patella syndrome to isolated nephropathy. Pediatr Nephrol. 2017;32:1845–50.
Sugiyama H, Yokoyama H, Sato H, Saito T, Kohda Y, Nishi S, et al. Japan Renal Biopsy Registry: the first nationwide, web-based, and prospective registry system of renal biopsies in Japan. Clin Exp Nephrol. 2011;15:493–503.
Sato U, Kitanaka S, Sekine T, Takahashi S, Ashida A, Igarashi T. Functional characterization of LMX1B mutations associated with nail-patella syndrome. Pediatr Res. 2005;57:783–8.
Fokkema IF, Taschner PE, Schaafsma GC, Celli J, Laros JF, den Dunnen JT. LOVD v.2.0: the next generation in gene variant databases. Hum Mutat. 2011;32:557–63.
Isojima T, Harita Y, Furuyama M, Sugawara N, Ishizuka K, Horita S, et al. LMX1B mutation with residual transcriptional activity as a cause of isolated glomerulopathy. Nephrol Dial Transpl. 2014;29:81–88.
Nakata T, Ishida R, Mihara Y, Fujii A, Inoue Y, Kusaba T, et al. Steroid-resistant nephrotic syndrome as the initial presentation of nail-patella syndrome: a case of a de novo LMX1B mutation. BMC Nephrol. 2017;18:100.
Bennett WM, Musgrave JE, Campbell RA, Elliot D, Cox R, Brooks RE, et al. The nephropathy of the nail-patella syndrome. Clinicopathologic analysis of 11 kindred. Am J Med. 1973;54:304–19.
Bongers EM, Gubler MC, Knoers NV. Nail-patella syndrome. Overview on clinical and molecular findings. Pediatr Nephrol. 2002;17:703–12.
Ghoumid J, Petit F, Holder-Espinasse M, Jourdain AS, Guerra J, Dieux-Coeslier A, et al. Nail-Patella Syndrome: clinical and molecular data in 55 families raising the hypothesis of a genetic heterogeneity. Eur J Hum Genet. 2016;24:44–50.
Dunston JA, Hamlington JD, Zaveri J, Sweeney E, Sibbring J, Tran C, et al. The human LMX1B gene: transcription unit, promoter, and pathogenic mutations. Genomics. 2004;84:565–76.
Bongers EM, de Wijs IJ, Marcelis C, Hoefsloot LH, Knoers NV. Identification of entire LMX1B gene deletions in nail patella syndrome: evidence for haploinsufficiency as the main pathogenic mechanism underlying dominant inheritance in man. Eur J Hum Genet. 2008;16:1240–4.
Gehring WJ, Qian YQ, Billeter M, Furukubo-Tokunaga K, Schier AF, Resendez-Perez D, et al. Homeodomain-DNA recognition. Cell. 1994;78:211–23.
Gehring WJ. The homeobox in perspective. Trends Biochem Sci. 1992;17:277–80.
Dreyer SD, Morello R, German MS, Zabel B, Winterpacht A, Lunstrum GP, et al. LMX1B transactivation and expression in nail-patella syndrome. Hum Mol Genet. 2000;9:1067–74.
Rose AS, Bradley AR, Valasatava Y, Duarte JM, Prlic A, Rose PW. NGL viewer: web-based molecular graphics for large complexes. Bioinformatics. 2018;34:3755–8.
Yin Y, Morgunova E, Jolma A, Kaasinen E, Sahu B, Kund-Sayeed S, et al. Impact of cytosine methylation on DNA binding specificities of human transcription factors. Science. 2017;356:eaaj2239.
Acknowledgements
This work was supported by Health and Labour Sciences Research Grants (H26-015 to YH, TI, SK, AA, and MH), and (H29-039 to YH) from the Ministry of Health, Labour and Welfare of Japan. We thank Dr Toshiyuki Fukasawa from Fukasawa Pediatric Clinic, Dr Seiji Tanaka from Kurume University, Dr Toshiharu Ueno from Toranomon Hospital, Dr Kunihiko Aya from Okayama University Medical School, Dr Yu Mihara from Kyoto Prefectural University of Medicine, Dr Hideaki Imamura from Miyazaki University, Dr Ema Takano from Nakaya Hospital, and Dr Noritaka Kawada from Osaka University for cooperating the survey. We also thank Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Funding
This work was supported by Health and Labour Sciences Research Grants (H26-015 to YH, TI, SK, AA, and MH), and (H29-039 to YH) from the Ministry of Health, Labour and Welfare of Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Harita, Y., Urae, S., Akashio, R. et al. Clinical and genetic characterization of nephropathy in patients with nail-patella syndrome. Eur J Hum Genet 28, 1414–1421 (2020). https://doi.org/10.1038/s41431-020-0655-3
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41431-020-0655-3