Abstract
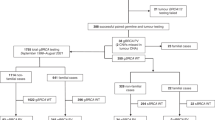
Poly(ADP-ribose) polymerase (PARP) inhibitors improve survival in BRCA-mutant high-grade serous ovarian carcinoma. As a result, germline and somatic BRCA1/2 testing has become standard practice in women diagnosed with ovarian cancer. We outline changes in testing and detection rates of germline BRCA1/2 pathogenic variants (PVs) in cases of non-mucinous epithelial ovarian cancer diagnosed during three eras, spanning 12 years, within the North West of England, and compare the uptake of cascade testing in families identified by oncology-led mainstreaming versus regional genetics clinics. Eras included: Period 1 (20% risk threshold for testing): between January 2007 and May 2013; Period 2 (10% risk threshold for testing): between June 2013 and October 2017 and; Period 3 (mainstream testing): between November 2017 and November 2019. A total of 1081 women underwent germline BRCA1/2 testing between January 2007 and November 2019 and 222 (20.5%) were found to have a PV. The monthly testing rate increased by 3.3-fold and 2.5-fold between Periods 1–2 and Periods 2–3, respectively. A similar incidence of germline BRCA1/2 PVs were detected in Period 2 (17.2%) and Period 3 (18.5%). Uptake of cascade testing from first-degree relatives was significantly lower in those women undergoing mainstream testing compared with those tested in regional genetics clinics (31.6% versus 47.3%, P = 0.038). Mainstream testing allows timely detection of germline BRCA1/2 status to select patients for PARP inhibitors, but shortfalls in the uptake of cascade testing in first-degree relatives requires optimisation to broaden benefits within families.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Kurman RJ, Carcangiu ML, Herrington CS, Young RH. WHO classification of tumours of female reproductive organs. 4th ed. International Agency for Research on Cancer (IARC); 2014.
Lheureux S, Gourley C, Vergote I, Oza AM. Epithelial ovarian cancer. Lancet. 2019;393:1240–53.
NCCN. National Comprehensive Cancer Network (NCCN) clinical practice guidelines in ovarian cancer. 2020. https://www.nccn.org/.
Ledermann JA, Raja FA, Fotopoulou C, Gonzalez-Martin A, Colombo N, Sessa C, et al. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi24–32.
Coleman RL, Fleming GF, Brady MF, Swisher EM, Steffensen KD, Friedlander M, et al. Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med. 2019;385:2403–15.
Gonzalez-Martin A, Pothuri B, Vergote I, DePont Christensen R, Graybill W, Mirza MR, et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N. Engl J Med. 2019;381:2391–402.
Moore K, Colombo N, Scambia G, Kim BG, Oaknin A, Friedlander M, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495–505.
Coleman RL, Oza AM, Lorusso D, Aghajanian C, Oaknin A, Dean A, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:1949–61.
Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, Redondo A, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375:2154–64.
Pujade-Lauraine E, Ledermann JA, Selle F, Gebski V, Penson RT, Oza AM, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1274–84.
Penson RT, Valencia RV, Cibula D, Colombo N, Leath CA III, Bidzinski M, et al. Olaparib versus nonplatinum chemotherapy in patients with platinum-sensitive relapsed ovarian cancer and a germline BRCA1/2 mutation (SOLO3): a randomized phase III trial. J Clin Oncol. 2020;38:1164–74.
Swisher EM, Lin KK, Oza AM, Scott CL, Giordano H, Sun J, et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 part 1): an international, multicentre, open-label, phase 2 trial. Lancet Oncol. 2017;18:75–87.
Moore KN, Secord AA, Geller MA, Miller DS, Cloven N, Fleming GF, et al. Niraparib monotherapy for late-line treatment of ovarian cancer (QUADRA): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019;20:636–48.
Huang KL, Mashl RJ, Wu Y, Ritter DI, Wang J, Oh C, et al. Pathogenic germline variants in 10,389 adult cancers. Cell. 2018;173:355–70.e14.
Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–15.
Rahman B, Lanceley A, Kristeleit RS, Ledermann JA, Lockley M, McCormack M, et al. Mainstreamed genetic testing for women with ovarian cancer: first-year experience. J Med Genet. 2019;56:195–8.
Plaskocinska I, Shipman H, Drummond J, Thompson E, Buchanan V, Newcombe B, et al. New paradigms for BRCA1/BRCA2 testing in women with ovarian cancer: results of the genetic testing in epithelial ovarian cancer (GTEOC) study. J Med Genet. 2016;53:655–61.
Rust K, Spiliopoulou P, Tang CY, Bell C, Stirling D, Phang T, et al. Routine germline BRCA1 and BRCA2 testing in patients with ovarian carcinoma: analysis of the Scottish real-life experience. BJOG. 2018;125:1451–8.
George A, Riddell D, Seal S, Talukdar S, Mahamdallie S, Ruark E, et al. Implementing rapid, robust, cost-effective, patient-centred, routine genetic testing in ovarian cancer patients. Sci Rep. 2016;6:29506.
Colombo N, Huang G, Scambia G, Chalas E, Pignata S, Fiorica J, et al. Evaluation of a streamlined oncologist-led BRCA mutation testing and counseling model for patients with ovarian cancer. J Clin Oncol. 2018;36:1300–7.
Kentwell M, Dow E, Antill Y, Wrede CD, McNally O, Higgs E, et al. Mainstreaming cancer genetics: a model integrating germline BRCA testing into routine ovarian cancer clinics. Gynecol Oncol. 2017;145:130–6.
Morgan RD, Burghel GJ, Flaum N, Bulman M, Clamp AR, Hasan J, et al. Prevalence of germline pathogenic BRCA1/2 variants in sequential epithelial ovarian cancer cases. J Med Genet. 2019;56:301–7.
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–24.
Evans DG, Harkness EF, Plaskocinska I, Wallace AJ, Clancy T, Woodward ER, et al. Pathology update to the Manchester Scoring System based on testing in over 4000 families. J Med Genet. 2017;54:674–81.
Schouten JP, McElgunn CJ, Waaijer R, Zwijnenburg D, Diepvens F, Pals G. Relative quantification of 40 nucleic acid sequences by multiplex ligation-dependent probe amplification. Nucleic Acids Res. 2002;30:e57.
Evans DG, Harkness EF, Plaskocinska I, Wallace AJ, Clancy T, Woodward ER, et al. Pathology update to the Manchester Scoring System based on testing in over 4000 families. J Med Genet. 2017;54:674–81.
Evans DG, Eccles DM, Rahman N, Young K, Bulman M, Amir E, et al. A new scoring system for the chances of identifying a BRCA1/2 mutation outperforms existing models including BRCAPRO. J Med Genet. 2004;41:474–80.
Alsop K, Fereday S, Meldrum C, deFazio A, Emmanuel C, George J, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol. 2012;30:2654–63.
Song H, Cicek MS, Dicks E, Harrington P, Ramus SJ, Cunningham JM, et al. The contribution of deleterious germline mutations in BRCA1, BRCA2 and the mismatch repair genes to ovarian cancer in the population. Hum Mol Genet. 2014;23:4703–9.
Flaum N, Crosbie EJ, Woodward ER, Lalloo F, Evans DGR. Challenging the believed proportion of ovarian cancer attributable to BRCA2 versus BRCA1 pathogenic variants. Eur J Cancer. 2020;124:88–90.
Wright S, Porteous M, Stirling D, Lawton J, Young O, Gourley C, et al. Patients’ views of treatment-focused genetic testing (TFGT): some lessons for the mainstreaming of BRCA1 and BRCA2 testing. J Genet Couns. 2018;27:1459–72.
Acknowledgements
ERW, EJC, EFH, and DGRE are supported by the all Manchester National Institute for Health Research Manchester Biomedical Research Centre (IS-BRC-1215-20007).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The germline BRCA1/2 database is approved by North Manchester Research Ethics Committee (08/H1006/77). All women included in this study provided informed verbal and/or written consent to undergo germline BRCA1/2 testing.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Flaum, N., Morgan, R.D., Burghel, G.J. et al. Mainstreaming germline BRCA1/2 testing in non-mucinous epithelial ovarian cancer in the North West of England. Eur J Hum Genet 28, 1541–1547 (2020). https://doi.org/10.1038/s41431-020-0692-y
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41431-020-0692-y
This article is cited by
-
Prevalence of cardiometabolic outcomes in women who underwent salpingo-oophorectomy to prevent hereditary breast and ovarian cancer: a meta-analysis
Familial Cancer (2025)
-
Cascade screening in HBOC and Lynch syndrome: guidelines and procedures in a UK centre
Familial Cancer (2024)
-
Experience of urologists, oncologists and nurse practitioners with mainstream genetic testing in metastatic prostate cancer
Prostate Cancer and Prostatic Diseases (2024)
-
Risk-reducing surgery for individuals with cancer-predisposing germline pathogenic variants and no personal cancer history: a review of current UK guidelines
British Journal of Cancer (2023)
-
A pilot study investigating feasibility of mainstreaming germline BRCA1 and BRCA2 testing in high-risk patients with breast and/or ovarian cancer in three tertiary Cancer Centres in Ireland
Familial Cancer (2023)