Abstract
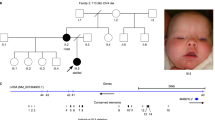
Defects in optic fissure closure can lead to congenital ocular coloboma. This ocular malformation, often associated with microphthalmia, is described in various clinical forms with different inheritance patterns and genetic heterogeneity. In recent times, the identification of an increased number of genes involved in numerous cellular functions has led to a better understanding in optic fissure closure mechanisms. Nevertheless, most of these genes are also involved in wider eye growth defects such as micro-anophthalmia, questioning the mechanisms controlling both extension and severity of optic fissure closure defects. However, some genes, such as FZD5, have only been so far identified in isolated coloboma. Thus, to estimate the frequency of implication of different ocular genes, we screened a cohort of 50 patients affected by ocular coloboma by using targeted sequencing of 119 genes involved in ocular development. This analysis revealed seven heterozygous (likely) pathogenic variants in RARB, MAB21L2, RBP4, TFAP2A, and FZD5. Surprisingly, three out of the seven variants detected herein were novel disease-causing variants in FZD5 identified in three unrelated families with dominant inheritance. Although molecular diagnosis rate remains relatively low in patients with ocular coloboma (14% (7/50) in this work), these results, however, highlight the importance of genetic screening, especially of FZD5, in such patients. Indeed, in our series, FZD5 variants represent half of the genetic causes, constituting 6% (3/50) of the patients who benefited from a molecular diagnosis. Our findings support the involvement of FZD5 in ocular coloboma and provide clues for screening this gene during current diagnostic procedures.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Patel A, Sowden JC. Genes and pathways in optic fissure closure. Semin Cell Dev Biol. 2019;91:55–65.
Graw J. Eye development. Curr Top Dev Biol. 2010;90:343–86.
Onwochei BC, Simon JW, Bateman JB, Couture KC, Mir E. Ocular colobomata. Sur Ophthalmol. 2000;45:175–94.
Gestri G, Bazin-Lopez N, Scholes C, Wilson SW. Cell behaviors during closure of the choroid fissure in the developing eye. Front Cell Neurosci. 2018;12:42.
Zagozewski JL, Zhang Q, Eisenstat DD. Genetic regulation of vertebrate eye development. Clin Genet. 2014;86:453–60.
Plaisancie J, Ceroni F, Holt R, Zazo Seco C, Calvas P, Chassaing N, et al. Genetics of anophthalmia and microphthalmia. Part 1: non-syndromic anophthalmia/microphthalmia. Hum Genet. 2019;138:799–830.
AS AL, Gregory-Evans CY, Gregory-Evans K. An update on the genetics of ocular coloboma. Hum Genet. 2019;138:865–80.
Shah SP, Taylor AE, Sowden JC, Ragge NK, Russell-Eggitt I, Rahi JS, et al. Anophthalmos, microphthalmos, and typical coloboma in the United Kingdom: a prospective study of incidence and risk. Invest Ophthalmol Vis Sci. 2011;52:558–64.
Chassaing N, Causse A, Vigouroux A, Delahaye A, Alessandri JL, Boespflug-Tanguy O, et al. Molecular findings and clinical data in a cohort of 150 patients with anophthalmia/microphthalmia. Clin Genet. 2014;86:326–34.
Liu C, Widen SA, Williamson KA, Ratnapriya R, Gerth-Kahlert C, Rainger J, et al. A secreted WNT-ligand-binding domain of FZD5 generated by a frameshift mutation causes autosomal dominant coloboma. Hum Mol Genet. 2016;25:1382–91.
Rainger J, Williamson KA, Soares DC, Truch J, Kurian D, Gillessen-Kaesbach G, et al. A recurrent de novo mutation in ACTG1 causes isolated ocular coloboma. Hum Mutat. 2017;38:942–6.
Fujimura N. WNT/beta-catenin signaling in vertebrate eye development. Front Cell Dev Biol. 2016;4:138.
Liu C, Nathans J. An essential role for frizzled 5 in mammalian ocular development. Development. 2008;135:3567–76.
Liu C, Bakeri H, Li T, Swaroop A. Regulation of retinal progenitor expansion by Frizzled receptors: implications for microphthalmia and retinal coloboma. Hum Mol Genet. 2012;21:1848–60.
Riviere JB, van Bon BW, Hoischen A, Kholmanskikh SS, O’Roak BJ, Gilissen C, et al. De novo mutations in the actin genes ACTB and ACTG1 cause Baraitser-Winter syndrome. Nat Genet. 2012;44:440–4.
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–24.
Srour M, Chitayat D, Caron V, Chassaing N, Bitoun P, Patry L, et al. Recessive and dominant mutations in retinoic acid receptor beta in cases with microphthalmia and diaphragmatic hernia. Am J Hum Genet. 2013;93:765–72.
Rainger J, Pehlivan D, Johansson S, Bengani H, Sanchez-Pulido L, Williamson KA, et al. Monoallelic and biallelic mutations in MAB21L2 cause a spectrum of major eye malformations. Am J Hum Genet. 2014;94:915–23.
Kalaskar VK, Alur RP, Li LK, Thomas JW, Sergeev YV, Blain D, et al. High-throughput custom capture sequencing identifies novel mutations in coloboma-associated genes: mutation in DNA-binding domain of retinoic acid receptor beta affects nuclear localization causing ocular coloboma. Hum Mutat. 2019;41:678–95.
Patel N, Khan AO, Alsahli S, Abdel-Salam G, Nowilaty SR, Mansour AM, et al. Genetic investigation of 93 families with microphthalmia or posterior microphthalmos. Clin Genet. 2018;93:1210–22.
Robitaille JM, Zheng B, Wallace K, Beis MJ, Tatlidil C, Yang J, et al. The role of Frizzled-4 mutations in familial exudative vitreoretinopathy and Coats disease. Brit J Ophthalmol. 2011;95:574–9.
Bang I, Kim HR, Beaven AH, Kim J, Ko SB, Lee GR, et al. Biophysical and functional characterization of Norrin signaling through Frizzled4. Proc Natl Acad Sci USA. 2018;115:8787–92.
Xu Q, Wang Y, Dabdoub A, Smallwood PM, Williams J, Woods C, et al. Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell. 2004;116:883–95.
Acknowledgements
We acknowledge generous support from the patients and their families as well as Claire Jeanton-Scaramouche for her precious help.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Aubert-Mucca, M., Pernin-Grandjean, J., Marchasson, S. et al. Confirmation of FZD5 implication in a cohort of 50 patients with ocular coloboma. Eur J Hum Genet 29, 131–140 (2021). https://doi.org/10.1038/s41431-020-0695-8
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41431-020-0695-8
This article is cited by
-
Intrafamilial variability of phenotype in CACNA2D4-associated retinal dysfunction: more or less
Documenta Ophthalmologica (2025)
-
Homozygosity for a hypomorphic mutation in frizzled class receptor 5 causes syndromic ocular coloboma with microcornea in humans
Human Genetics (2024)
-
Deletion upstream of MAB21L2 highlights the importance of evolutionarily conserved non-coding sequences for eye development
Nature Communications (2024)