Abstract
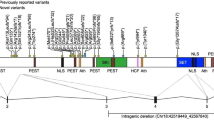
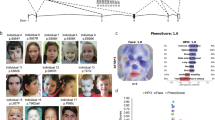
SETBP1 haploinsufficiency disorder (MIM#616078) is caused by haploinsufficiency of SETBP1 on chromosome 18q12.3, but there has not yet been any systematic evaluation of the major features of this monogenic syndrome, assessing penetrance and expressivity. We describe the first comprehensive study to delineate the associated clinical phenotype, with findings from 34 individuals, including 24 novel cases, all of whom have a SETBP1 loss-of-function variant or single (coding) gene deletion, confirmed by molecular diagnostics. The most commonly reported clinical features included mild motor developmental delay, speech impairment, intellectual disability, hypotonia, vision impairment, attention/concentration deficits, and hyperactivity. Although there is a mild overlap in certain facial features, the disorder does not lead to a distinctive recognizable facial gestalt. As well as providing insight into the clinical spectrum of SETBP1 haploinsufficiency disorder, this reports puts forward care recommendations for patient management.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hoischen A, van Bon BW, Gilissen C, Arts P, van Lier B, Steehouwer M, et al. De novo mutations of SETBP1 cause Schinzel-Giedion syndrome. Nat Genet. 2010;42:483–5.
Tinkle Brad TB, Christianson CA, Schorry EK, Webb T, Hopkin RJ. Long-term survival in a patient with del(18)(q12.2q21.1). Am J Med Genet Part A. 2003;119A:66–70.
Kotzot Dieter D, Kotzot D, Haberlandt E, Fauth C, Baumgartner S, Scholl-Bürgi S, et al. Del(18)(q12.2q21.1) caused by a paternal sister chromatid rearrangement in a developmentally delayed girl. Am J Med Genet Part A. 2005;135:304–7.
Feenstra I, Vissers LE, Orsel M, van Kessel AG, Brunner HG, Veltman JA, et al. Genotype-phenotype mapping of chromosome 18q deletions by high-resolution array CGH: an update of the phenotypic map. Am J Med Genet Part A. 2007;143A:1858–67.
Bouquillon S, Andrieux J, Landais E, Duban-Bedu B, Boidein F, Lenne B, et al. A 5.3Mb deletion in chromosome 18q12.3 as the smallest region of overlap in two patients with expressive speech delay. Eur J Med Genet. 2011;54:194–7.
Buysse K, Menten B, Oostra A, Tavernier S, Mortier GR, Speleman F. Delineation of a critical region on chromosome 18 for the del(18)(q12.2q21.1) syndrome. Am J Med Genet Part A. 2008;146A:1330–4.
Cody JD, Sebold C, Malik A, Heard P, Carter E, Crandall A, et al. Recurrent interstitial deletions of proximal 18q: a new syndrome involving expressive speech delay. Am J Med Genet Part A. 2007;143A:1181–90.
Filges I, Shimojima K, Okamoto N, Röthlisberger B, Weber P, Huber AR, et al. Reduced expression by SETBP1 haploinsufficiency causes developmental and expressive language delay indicating a phenotype distinct from Schinzel-Giedion syndrome. J Med Genet. 2011;48:117–22.
Marseglia G, Scordo MR, Pescucci C, Nannetti G, Biagini E, Scandurra V, et al. 372 kb microdeletion in 18q12.3 causing SETBP1 haploinsufficiency associated with mild mental retardation and expressive speech impairment. Eur J Med Genet. 2012;55:216–21.
Eising E, Carrion-Castillo A, Vino A, Strand EA, Jakielski KJ, Scerri TS, et al. A set of regulatory genes co-expressed in embryonic human brain is implicated in disrupted speech development. Mol Psychiatry. 2019;24:1065–78.
Hildebrand MS, Jackson VE, Scerri TS, Van Reyk O, Coleman M, Braden RO, et al. Severe childhood speech disorder: gene discovery highlights transcriptional dysregulation. Neurology. 2020;94:e2148–67.
Hamdan FF, Srour M, Capo-Chichi JM, Daoud H, Nassif C, Patry L, et al. De novo mutations in moderate or severe intellectual disability. PLoS Genet. 2014;10:e1004772.
Rauch A, Wieczorek D, Graf E, Wieland T, Endele S, Schwarzmayr T, et al. Range of genetic mutations associated with severe non-syndromic sporadic intellectual disability: an exome sequencing study. Lancet. 2012;380:1674–82.
Coe BP, Witherspoon K, Rosenfeld JA, van Bon BW, Vulto-van Silfhout AT, Bosco P, et al. Refining analyses of copy number variation identifies specific genes associated with developmental delay. Nat Genet. 2014;46:1063–71.
Fokkema IFAC, Taschner PEM, Schaafsma GCP, Celli J, Laros JFJ, den Dunnen JT. LOVD v.2.0: the next generation in gene variant databases. Hum Mutat. 2011;32:557–63.
Sobreira N, Schiettecatte F, Valle D, Hamosh A. GeneMatcher: a matching tool for connecting investigators with an interest in the same gene. Hum Mutat. 2015;36:928–30.
WHO. Motor Development Study: windows of achievement for six gross motor development milestones. Acta Paediatr Suppl. 2006;450:86–95.
Piazza R, Magistroni V, Redaelli S, Mauri M, Massimino L, Sessa A, et al. SETBP1 induces transcription of a network of development genes by acting as an epigenetic hub. Nat Commun. 2018;9:2192.
Coban-Akdemir Z, White JJ, Song X, Jhangiani SN, Fatih JM, Gambin T, et al. Identifying Genes Whose Mutant Transcripts Cause Dominant Disease Traits by Potential Gain-of-Function Alleles. Am J Hum Genet. 2018;103:171–87.
Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–91.
Bieber T. Atopic Dermatitis. N Engl J Med. 2008;358:1483–94.
Acknowledgements
The authors are grateful to all the patients and their parents who collaborated on our research and gave their consent to share the clinical data and/or photos. SEF & MMK are supported by the Max Planck Society.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Jansen, N.A., Braden, R.O., Srivastava, S. et al. Clinical delineation of SETBP1 haploinsufficiency disorder. Eur J Hum Genet 29, 1198–1205 (2021). https://doi.org/10.1038/s41431-021-00888-9
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41431-021-00888-9
This article is cited by
-
Schinzel-Giedion syndrome: communication, feeding and motor skills in 16 individuals
Neurogenetics (2025)
-
SETBP1 Contributes to the Progression of Neuroblastoma Through Regulating EPHA7 Expression
Iranian Journal of Science (2025)
-
SETBP1 variants outside the degron disrupt DNA-binding, transcription and neuronal differentiation capacity to cause a heterogeneous neurodevelopmental disorder
Nature Communications (2025)
-
Identifying SETBP1 haploinsufficiency molecular pathways to improve patient diagnosis using induced pluripotent stem cells and neural disease modelling
Molecular Autism (2024)
-
Structural rearrangements as a recurrent pathogenic mechanism for SETBP1 haploinsufficiency
Human Genomics (2024)