Abstract
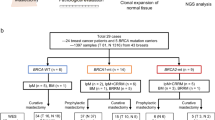
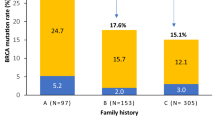
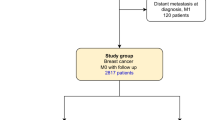
Women with pathogenic germline BRCA1 or BRCA2 variants have a higher risk of breast cancer than in the general population. International guidelines recommend specific clinical and radiological breast follow-up. This specific breast screening program has already been shown to be of clinical benefit, but no information is available concerning the use of prognostic factors or specific survival to guide follow-up decisions. We evaluated “high-risk” screening in a retrospective single-center study of 520 women carrying pathogenic germline variants of the BRCA1 or BRCA2 gene treated for breast cancer between January 2000 and December 2016. We compared two groups of women: the incidental breast cancer group (IBCG) were followed before breast cancer diagnosis (N = 103), whereas the prevalent breast cancer group (PBCG) (N = 417) had no specific follow-up for high risk before breast cancer diagnosis. Breast cancers were diagnosed at an earlier stage in the IBCG than in the PBCG: T0 in 64% versus 19% of tumors, (p < 0.00001), and N0 in 90% vs. 75% (p < 0.00001), respectively. Treatment differed significantly between the 2 groups: less neoadjuvant chemotherapy (7.1% vs. 28.5%, p < 0.00001), adjuvant chemotherapy (47.7% vs. 61.9%, p = 0.004) and more mastectomies (60% vs. 42% p < 0.0001) in the IBCG vs PBCG groups respectively. Overall and breast cancer-specific mortality were similar between the two groups. However, the patients in the IBCG had a significantly longer metastasis-free survival than those in the PBCG, at three years (96.9% [95% CI 93.5–100] vs. 92.30% [95% CI 89.8–94.9]; p = 0.02), suggesting a possible long-term survival advantage.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
The clinical data analyzed during the current study are available from the corresponding author on reasonable request.
References
Kuchenbaecker KB, Hopper JL, Barnes DR, Phillips K-A, Mooij TM, Roos-Blom M-J, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017;317:2402–16. 20.
National Collaborating Centre for Cancer (UK). Familial Breast Cancer: Classification and Care of People at Risk of Familial Breast Cancer and Management of Breast Cancer and Related Risks in People with a Family History of Breast Cancer [Internet]. Cardiff (UK): National Collaborating Centre for Cancer (UK); 2013 [cited 2020 Feb 18]. (National Institute for Health and Clinical Excellence: Guidance). Available from: http://www.ncbi.nlm.nih.gov/books/NBK247567/.
Paluch-Shimon S, Cardoso F, Sessa C, Balmana J, Cardoso MJ, Gilbert F, et al. Prevention and screening in BRCA mutation carriers and other breast/ovarian hereditary cancer syndromes: ESMO Clinical Practice Guidelines for cancer prevention and screening. Ann Oncol. 2016;27:v103–10.
Daly MB, Pilarski R, Yurgelun MB, Berry MP, Buys SS, Dickson P, et al. NCCN Guidelines Insights: Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 1.2020 [Internet]. J Natl Compr Cancer Netw.: JNCCN. 2020 [cited 2020 Dec 8]. Available from: https://pubmed.ncbi.nlm.nih.gov/32259785/.
Synthèse - Femmes porteuses d’une mutation de BRCA1 ou BRCA2/Détection précoce du cancer du sein et des annexes et stratégies de réduction du risque - Ref: RECOBRCASYNTH17 | Institut National Du Cancer [Internet]. [cited 2017 Aug 15]. Available from: http://www.e-cancer.fr/Expertises-et-publications/Catalogue-des-publications/Synthese-Femmes-porteuses-d-une-mutation-de-BRCA1-ou-BRCA2-Detection-precoce-du-cancer-du-sein-et-des-annexes-et-strategies-de-reduction-du-risque.
Chirurgie prophylactique des cancers avec prédisposition génétique - Ref: RECOCHIRPRO09 [Internet]. [cited 2020 Feb 25]. Available from: https://www.e-cancer.fr/Expertises-et-publications/Catalogue-des-publications/Chirurgie-prophylactique-des-cancers-avec-predisposition-genetique.
Jakub JW, Peled AW, Gray RJ, Greenup RA, Kiluk JV, Sacchini V, et al. Oncologic safety of prophylactic nipple-sparing mastectomy in a population with BRCA mutations: a multi-institutional study. JAMA Surg. 2018;153:123–9.
Hartmann LC, Lindor NM. The role of risk-reducing surgery in hereditary breast and ovarian cancer. N. Engl J Med. 2016;374(Feb):454–68.
Heemskerk-Gerritsen BAM, Jager A, Koppert LB, Obdeijn AI-M, Collée M, Meijers-Heijboer HEJ, et al. Survival after bilateral risk-reducing mastectomy in healthy BRCA1 and BRCA2 mutation carriers. Breast Cancer Res Treat. 2019;177(Oct):723–33.
Kurian AW, Sigal BM, Plevritis SK. Survival analysis of cancer risk reduction strategies for BRCA1/2 mutation carriers. J Clin Oncol. 2010;28(Jan):222–31.
Rojas KE, Butler E, Gutierrez J, Kwait R, Laprise J, Wilbur JS, et al. Choosing high-risk screening vs. surgery and the effect of treatment modality on anxiety and breast-specific sensuality in BRCA mutation carriers. Gland Surg. 2019;8(Jun):249–57.
Krammer J, Pinker-Domenig K, Robson ME, Gönen M, Bernard-Davila B, Morris EA, et al. Breast cancer detection and tumor characteristics in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res Treat. 2017;163(Jun):565–71.
Phi X-A, Saadatmand S, De Bock GH, Warner E, Sardanelli F, Leach MO, et al. Contribution of mammography to MRI screening in BRCA mutation carriers by BRCA status and age: individual patient data meta-analysis. Br J Cancer. 2016;114(Mar):631–7.
Evans DG, Gareth ED, Kesavan N, Nisha K, Lim Y, Yit L, et al. MRI breast screening in high-risk women: cancer detection and survival analysis. Breast Cancer Res Treat. 2014;145(Jun):663–72.
Passaperuma K, Warner E, Causer PA, Hill KA, Messner S, Wong JW, et al. Long-term results of screening with magnetic resonance imaging in women with BRCA mutations. Br J Cancer. 2012;107(Jun):24–30.
Warner E, Hill K, Causer P, Plewes D, Jong R, Yaffe M, et al. Prospective study of breast cancer incidence in women with a BRCA1 or BRCA2 mutation under surveillance with and without magnetic resonance imaging. J Clin Oncol. 2011;29(May):1664–9.
Leach MO, Boggis CRM, Dixon AK, Easton DF, Eeles RA, Evans DGR, et al. Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: a prospective multicentre cohort study (MARIBS). Lancet. 2005;365(May):1769–78.
Kriege M, Brekelmans CTM, Boetes C, Besnard PE, Zonderland HM, Obdeijn IM, et al. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N. Engl J Med. 2004;351(Jul):427–37.
Murakami W, Tozaki M, Nakamura S, Ide Y, Inuzuka M, Hirota Y, et al. The clinical impact of MRI screening for BRCA mutation carriers: the first report in Japan. Breast Cancer. 2019;26(Sep):552–61.
Chéreau E, Uzan C, Balleyguier C, Chevalier J, de Paillerets BB, Caron O, et al. Characteristics, treatment, and outcome of breast cancers diagnosed in BRCA1 and BRCA2 gene mutation carriers in intensive screening programs including magnetic resonance imaging. Clin Breast Cancer. 2010;10(Apr):113–8.
Saadatmand S, Geuzinge HA, Rutgers EJT, Mann RM, de Roy van Zuidewijn DBW, Zonderland HM, et al. MRI versus mammography for breast cancer screening in women with familial risk (FaMRIsc): a multicentre, randomised, controlled trial. Lancet Oncol. 2019;20(Aug):1136–47.
Riedl CC, Luft N, Bernhart C, Weber M, Bernathova M, Tea M-KM, et al. Triple-modality screening trial for familial breast cancer underlines the importance of magnetic resonance imaging and questions the role of mammography and ultrasound regardless of patient mutation status, age, and breast density. J Clin Oncol. 2015;33(Apr):1128–35.
Bick U, Engel C, Krug B, Heindel W, Fallenberg EM, Rhiem K, et al. High-risk breast cancer surveillance with MRI: 10-year experience from the German consortium for hereditary breast and ovarian cancer. Breast Cancer Res Treat. 2019;175(May):217–28.
Evans DG, Howell SJ, Gandhi A, van Veen EM, Woodward ER, Harvey J, et al. Breast cancer incidence and early diagnosis in a family history risk and prevention clinic: 33-year experience in 14,311 women. Breast Cancer Res Treat. 2021;189(Oct):677–87.
Møller P, Stormorken A, Jonsrud C, Holmen MM, Hagen AI, Clark N, et al. Survival of patients with BRCA1-associated breast cancer diagnosed in an MRI-based surveillance program. Breast Cancer Res Treat. 2013;139(May):155–61.
Saadatmand S, Obdeijn I-M, Rutgers EJ, Oosterwijk JC, Tollenaar RA, Woldringh GH, et al. Survival benefit in women with BRCA1 mutation or familial risk in the MRI screening study (MRISC). Int J Cancer. 2015;137(Oct):1729–38.
Hadar T, Mor P, Amit G, Lieberman S, Gekhtman D, Rabinovitch R, et al. Presymptomatic awareness of germline pathogenic BRCA variants and associated outcomes in women with breast cancer. JAMA Oncol. 2020;6:1460–3.
Tubiana M, Koscielny S. Natural history of human breast cancer: recent data and clinical implications. Breast Cancer Res Treat. 1991;18(Aug):125–40.
Ding L, Su Y, Fassl A, Hinohara K, Qiu X, Harper NW, et al. Perturbed myoepithelial cell differentiation in BRCA mutation carriers and in ductal carcinoma in situ. Nat Commun. 2019;10:4182.
Ter Welle-Butalid MEE, Vriens IJHI, Derhaag JGJ, Leter EME, de Die-Smulders CEC, Smidt MM, et al. Counseling young women with early breast cancer on fertility preservation. J Assist Reprod Genet. 2019;36(Dec):2593–604.
Terada M, Yoshimura A, Sawaki M, Hattori M, Naomi G, Kotani H, et al. Patient-reported outcomes and objective assessments with arm measurement and bioimpedance analysis for lymphedema among breast cancer survivors. Breast Cancer Res Treat. 2020;179(Jan):91–100.
Friedlaender A, Vuilleumier A, Viassolo V, Ayme A, De Talhouet S, Combes J-D, et al. BRCA1/BRCA2 germline mutations and chemotherapy-related hematological toxicity in breast cancer patients. Breast Cancer Res Treat. 2019;174(Apr):775–83.
Iqbal J, Nussenzweig A, Lubinski J, Byrski T, Eisen A, Bordeleau L, et al. The incidence of leukaemia in women with BRCA1 and BRCA2 mutations: an International Prospective Cohort Study. Br J Cancer. 2016;114:1160–4.
Churpek JE, Marquez R, Neistadt B, Claussen K, Lee MK, Churpek MM, et al. Inherited mutations in cancer susceptibility genes are common among survivors of breast cancer who develop therapy-related leukemia. Cancer. 2016;122(Jan):304–11.
Tomao F, Musacchio L, Di Mauro F, Boccia SM, Di Donato V, Giancotti A, et al. Is BRCA mutational status a predictor of platinum-based chemotherapy related hematologic toxicity in high-grade serous ovarian cancer patients? Gynecol Oncol. 2019;154:138–43.
Edmonds CE, Lamb LR, Mercaldo SF, Sippo DA, Burk KS, Lehman CD. Frequency and cancer yield of BI-RADS Category 3 lesions detected at high-risk screening breast MRI. AJR Am J Roentgenol. 2020;214(Feb):240–8.
Chikarmane SA, Tai R, Meyer JE, Giess CS. Prevalence and predictive value of BI-RADS 3, 4, and 5 lesions detected on breast MRI: correlation with study indication. Acad Radiol. 2017;24(Apr):435–41.
Lourenco AP, Donegan L, Khalil H, Mainiero MB. Improving outcomes of screening breast MRI with practice evolution: initial clinical experience with 3T compared to 1.5T. J Magn Reson Imaging. 2014;39(Mar):535–9.
Panigrahi B, Harvey SC, Mullen LA, Falomo E, Di Carlo P, Lee B, et al. Characteristics and outcomes of BI-RADS 3 lesions on breast MRI. Clin Breast Cancer. 2019;19(Feb):e152–9.
Vreemann S, van Zelst JCM, Schlooz-Vries M, Bult P, Hoogerbrugge N, Karssemeijer N, et al. The added value of mammography in different age-groups of women with and without BRCA mutation screened with breast MRI. Breast Cancer Res. 2018;20(Aug):84.
Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N. Engl J Med. 2002;347(Oct):1227–32.
Valachis A, Nearchou AD, Lind P. Surgical management of breast cancer in BRCA-mutation carriers: a systematic review and meta-analysis. Breast Cancer Res Treat. 2014;144(Apr):443–55.
van den Broek AJ, van’t Veer LJ, Hooning MJ, Cornelissen S, Broeks A, Rutgers EJ, et al. Impact of age at primary breast cancer on contralateral breast cancer risk in BRCA1/2 mutation carriers. J Clin Oncol. 2016;34(Feb):409–18.
Copson ER, Maishman TC, Tapper WJ, Cutress RI, Greville-Heygate S, Altman DG, et al. Germline BRCA mutation and outcome in young-onset breast cancer (POSH): a prospective cohort study. Lancet Oncol. 2018;19(Feb):169–80.
Waks AG, Winer EP. Breast cancer treatment: a review. JAMA. 2019;321(Jan):288–300.
Alegre N, Perre PV, Bignon YJ, Michel A, Galibert V, Mophawe O, et al. Psychosocial and clinical factors of probands impacting intrafamilial disclosure and uptake of genetic testing among families with BRCA1/2 or MMR gene mutations. Psychooncology. 2019;28:1679–86.
Pujol P, Barberis M, Beer P, Friedman E, Piulats JM, Capoluongo ED, et al. Clinical practice guidelines for BRCA1 and BRCA2 genetic testing. Eur J Cancer. 2021;146(Mar):30–47.
Dhar SU, Cooper HP, Wang T, Parks B, Staggs SA, Hilsenbeck S, et al. Significant differences among physician specialties in management recommendations of BRCA1 mutation carriers. Breast Cancer Res Treat. 2011;129(Aug):221–7.
Eccles BK, Copson E, Maishman T, Abraham JE, Eccles DM. Understanding of BRCA VUS genetic results by breast cancer specialists. BMC Cancer. 2015;15(Nov):936.
Funding
This project has not received specific funding.
Author information
Authors and Affiliations
Contributions
Study design: CS, SMH, EMF, DSL. Data collection: CS, SMH, EMF. Data analysis: EMF, MC. Data interpretation: CS, SMH, EMF. Writing: CS, SMH, EMF. Extensive revision of the manuscript: CS, SMH, MC, CM, PC, FR, ML, EG, AD, NC, SF, FC, DSL, EMF.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The study was approved by the Breast Cancer Study Group of Institut Curie and was conducted according to institutional and ethical rules concerning research on tissue specimens and patients.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saule, C., Menu-Hespel, S., Carton, M. et al. Prevalent versus incident breast cancers: benefits of clinical and radiological monitoring in women with pathogenic BRCA1/2 variants. Eur J Hum Genet 30, 1060–1066 (2022). https://doi.org/10.1038/s41431-022-01049-2
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41431-022-01049-2
This article is cited by
-
Guidelines, guidelines everywhere—and still I’m not sure what to do
European Journal of Human Genetics (2022)