Abstract
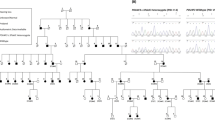
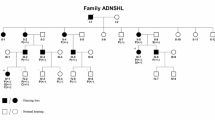
Hearing impairment (HI) is a significant health concern globally, influenced by genetic and environmental factors. We had identified a homozygous pathogenic variant in POLD3 in a Lebanese patient with an autosomal congenital recessive syndromic hearing loss (MIM#620869). This variant was found at heterozygous state in the parents, who developed progressive hearing impairment around age 40. We conducted a thorough clinical and genetic assessment of sixteen family members, including physical exams, audiometry and vestibular function evaluations. Additionally, gene expression analysis of the Pold3 gene was performed in mice using RNAscope. Twelve individuals were heterozygous for the variant in POLD3, of whom eight showed bilateral adult-onset HI, typically starting around ages 40–50, and two older patients displaying unilateral vestibular weakness. Additionally, two carriers of the variant developed cancer at an early age. RNAscope confirmed Pold3 expression in auditory and vestibular neurons. Exome sequencing analysis excluded the presence of pathogenic variants in any known hearing impairment or cancer predisposition genes. We present herein, for the first time, evidence of a heterozygous pathogenic POLD3 variant associated with a novel form of autosomal dominant progressive adult-onset hearing and vestibular impairments. We also highlight the necessity for further exploration of the role of POLD3 in cancer predisposition.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The datasets used and analyzed during the current study are available from the corresponding author upon a reasonable request.
References
Jasper KM, Jamshidi A, Reilly BK. Pediatric otolaryngology, molecular diagnosis of hereditary hearing loss: next-generation sequencing approach. Curr Opin Otolaryngol Head Neck Surg. 2015;23:480–4.
Lanzieri TM, Lu T, Bennett MV, Hintz SR, Sugerman DE, Dollard SC, et al. Early childhood outcomes of NICU graduates with cytomegalovirus infection in California. Birth Defects Res. 2023;115:1093–100.
Arvin B, Prepageran N, Raman R. High frequency presbycusis”–is there an earlier onset? Indian J Otolaryngol Head Neck Surg. 2013;65:480–4.
Wolber LE, Steves CJ, Spector TD, Williams FMK. Hearing ability with age in northern European women: a new web-based approach to genetic studies. PLoS ONE. 2012;7:e35500.
Gates GA, Couropmitree NN, Myers RH. Genetic associations in age-related hearing thresholds. Arch Otolaryngol Neck Surg. 1999;125:654.
Demeester K, Van Wieringen A, Hendrickx JJ, Topsakal V, Huyghe J, Fransen E, et al. Heritability of audiometric shape parameters and familial aggregation of presbycusis in an elderly Flemish population. Hear Res. 2010;265:1–10.
Huang Q, Tang J. Age-related hearing loss or presbycusis. Eur Arch Otorhinolaryngol. 2010;267:1179–91.
Wells HRR, Freidin MB, Zainul Abidin FN, Payton A, Dawes P, Munro KJ, et al. GWAS identifies 44 independent associated genomic loci for self-reported adult hearing difficulty in UK Biobank. Am J Hum Genet. 2019;105:788–802.
Ninoyu Y, Friedman RA. The genetic landscape of age-related hearing loss. Trends Genet. 2024;40:228–37.
Zhang L, Gao Y, Zhang R, Sun F, Cheng C, Qian F, et al. THOC1 deficiency leads to late-onset nonsyndromic hearing loss through p53-mediated hair cell apoptosis. PLOS Genet. 2020;16:e1008953.
Boucher S, Tai FWJ, Delmaghani S, Lelli A, Singh-Estivalet A, Dupont T, et al. Ultrarare heterozygous pathogenic variants of genes causing dominant forms of early-onset deafness underlie severe presbycusis. Proc Natl Acad Sci. 2020;117:31278–89.
Aldè M, Cantarella G, Zanetti D, Pignataro L, La Mantia I, Maiolino L, et al. Autosomal dominant non-syndromic hearing loss (DFNA): a comprehensive narrative review. Biomedicines. 2023;11:1616.
Usami SI, Isaka Y, Miyagawa M, Nishio SY. Variants in CDH23 cause a broad spectrum of hearing loss: from non-syndromic to syndromic hearing loss as well as from congenital to age-related hearing loss. Hum Genet. 2022;141:903–14.
Lim HD, Lee SM, Yun YJ, Lee DH, Lee JH, Oh SH, et al. WFS1 autosomal dominant variants linked with hearing loss: update on structural analysis and cochlear implant outcome. BMC Med Genom. 2023;16:79.
Velde HM, Huizenga XJJ, Yntema HG, Haer-Wigman L, Beynon AJ, Oostrik J, et al. Genotype and phenotype analyses of a Novel WFS1 Variant (c.2512C>T p.(Pro838Ser)) Associated with DFNA6/14/38. Genes. 2023;14:457.
Kytövuori L, Hannula S, Mäki-Torkko E, Sorri M, Majamaa K. A nonsynonymous mutation in the WFS1 gene in a Finnish family with age-related hearing impairment. Hear Res. 2017;355:97–101.
Heyne HO, Karjalainen J, Karczewski KJ, Lemmelä SM, Zhou W, FinnGen, et al. Mono- and biallelic variant effects on disease at biobank scale. Nature. 2023;613:519–25.
Hames A, Khan S, Gilliland C, Goldman L, Lo HW, Magda K, et al. Carriers of autosomal recessive conditions: are they really ‘unaffected? J Med Genet. 2024;61:1–7.
Mehawej C, Chouery E, Azar-Atallah S, Shebaby W, Delague V, Mansour I, et al. POLD3 deficiency is associated with severe combined immunodeficiency, neurodevelopmental delay, and hearing impairment. Clin Immunol. 2023;251:109326.
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–24.
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–82.
Jones SM, Jones TA, Johnson KR, Yu H, Erway LC, Zheng QY. A comparison of vestibular and auditory phenotypes in inbred mouse strains. Brain Res. 2006;1091:40–6.
Iwasaki S, Takai Y, Ito K, Murofushi T. Abnormal vestibular evoked myogenic potentials in the presence of normal caloric responses. Otol Neurotol. 2005;26:1196–9.
Young Y, Wu C, Wu C. Augmentation of vestibular evoked myogenic potentials: an indication for distended saccular hydrops. Laryngoscope. 2002;112:509–12.
Wang F, Flanagan J, Su N, Wang LC, Bui S, Nielson A, et al. RNAscope. J Mol Diagn. 2012;14:22–9.
Grandi FC, De Tomasi L, Mustapha M. Corrigendum: single-cell RNA analysis of type I spiral ganglion neurons reveals a Lmx1a population in the Cochlea. Front Mol Neurosci. 2021;14:686790.
Rayner E, Van Gool IC, Palles C, Kearsey SE, Bosse T, Tomlinson I, et al. A panoply of errors: polymerase proofreading domain mutations in cancer. Nat Rev Cancer. 2016;16:71–81.
Conde CD, Petronczki ÖY, Baris S, Willmann KL, Girardi E, Salzer E, et al. Polymerase δ deficiency causes syndromic immunodeficiency with replicative stress. J Clin Invest. 2019;129:4194–206.
Oh D, Matsumoto Y, Kitajiri S, Kim NKD, Kim MY, Kim AR, et al. POLD1 variants leading to reduced polymerase activity can cause hearing loss without syndromic features. Hum Mutat. 2020;41:913–20.
Elouej S, Beleza-Meireles A, Caswell R, Colclough K, Ellard S, Desvignes JP, et al. Exome sequencing reveals a de novo POLD1 mutation causing phenotypic variability in mandibular hypoplasia, deafness, progeroid features, and lipodystrophy syndrome (MDPL). Metabolism. 2017;71:213–25.
Cui Y, Keles S, Charbonnier LM, Julé AM, Henderson L, Celik SC, et al. Combined immunodeficiency caused by a loss-of-function mutation in DNA polymerase delta 1. J Allergy Clin Immunol. 2020;145:391–401.e8.
Lai JP, Liu YC, Alimchandani M, Liu Q, Aung PP, Matsuda K, et al. The influence of DNA repair on neurological degeneration, cachexia, skin cancer and internal neoplasms: autopsy report of four xeroderma pigmentosum patients (XP-A, XP-C and XP-D). Acta Neuropathol Commun. 2013;1:4.
Spoor M, Nagtegaal AP, Ridwan Y, Borgesius NZ, Van Alphen B, Van Der Pluijm I, et al. Accelerated loss of hearing and vision in the DNA-repair deficient Ercc1δ/− mouse. Mech Ageing Dev. 2012;133:59–67.
Paplou V, Schubert NMA, Pyott SJ. Age-related changes in the cochlea and vestibule: shared patterns and processes. Front Neurosci. 2021;15:680856.
Gallego-Martinez A, Espinosa-Sanchez JM, Lopez-Escamez JA. Genetic contribution to vestibular diseases. J Neurol. 2018;265:29–34.
Frejo L, Giegling I, Teggi R, Lopez-Escamez JA, Rujescu D. Genetics of vestibular disorders: pathophysiological insights. J Neurol. 2016;263:45–53.
Yang J, Liu Y, Zhang Q, Yu L, Murofushi T, Jahn K, et al. Editorial: vestibular disorders in children. Front Neurol. 2023;14:1142504.
Shrestha BR, Chia C, Wu L, Kujawa SG, Liberman MC, Goodrich LV. Sensory neuron diversity in the inner ear is shaped by activity. Cell. 2018;174:1229–46.e17.
DeStefano AL, Gates GA, Heard-Costa N, Myers RH, Baldwin CT. Genomewide linkage analysis to presbycusis in the framingham heart study. Arch Otolaryngol Neck Surg. 2003;129:285.
Ahmadmehrabi S, Brant J, Epstein DJ, Ruckenstein MJ, Rader DJ. Genetics of postlingual sensorineural hearing loss. Laryngoscope. 2021;131:401–9.
Imbaud Genieys S. Troubles de l’équilibre chez le sujet âgé. Ann Otolaryngol Chir Cervico-Facial. 2007;124:189–96.
Iwasaki S, Yamasoba T. Dizziness and imbalance in the elderly: age-related decline in the vestibular system. Aging Dis. 2015;6:38.
Janky KL, Patterson J, Shepard N, Thomas M, Barin K, Creutz T, et al. Video Head Impulse Test (vHIT): The role of corrective saccades in identifying patients with vestibular loss. Otol Neurotol. 2018;39:467–73.
Tumini E, Barroso S, -Calero CP, Aguilera A. Roles of human POLD1 and POLD3 in genome stability. Sci Rep. 2016;6:38873.
Fuchs J, Cheblal A, Gasser SM. Underappreciated roles of DNA polymerase δ in replication stress survival. Trends Genet. 2021;37:476–87.
Zhou Z, Wang L, Ge F, Gong P, Wang H, Wang F, et al. Pold3 is required for genomic stability and telomere integrity in embryonic stem cells and meiosis. Nucleic Acids Res. 2018;46:3468–86.
Zhao S, Wei C, Tang H, Ding H, Han B, Chen S, et al. Elevated DNA polymerase delta 1 expression correlates with tumor progression and immunosuppressive tumor microenvironment in hepatocellular carcinoma. Front Oncol. 2021;11:736363.
Elsayed FA, Kets CM, Ruano D, Van Den Akker B, Mensenkamp AR, Schrumpf M, et al. Germline variants in POLE are associated with early onset mismatch repair deficient colorectal cancer. Eur J Hum Genet. 2015;23:1080–4.
Bergen G, Stevens MR, Burns ER. Falls and fall injuries among adults aged ≥65 years — United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65:993–8.
Vaishya R, Vaish A. Falls in older adults are serious. Indian J Orthop. 2020;54:69–74.
Acknowledgements
We express our deepest gratitude and sympathy to the family for their full cooperation throughout the study.
Funding
This study was funded by the President Intramural Research Fund (PIRF) of the Lebanese American University (PIRF- I0004). Authors did not receive any personal funding. Medical Research Council: MR/X012077/1 (LMF). Medical Research Council: MR/S002510/1 (MMustapha). Imaging work was performed at the Wolfson Light Microscopy Facility, using the Zeiss LSM 980 Airyscan 2 confocal microscope (MRC grant MR/X012077/1).
Author information
Authors and Affiliations
Contributions
EC, CM, MM and AM conceived, designed the study, performed data interpretation, and wrote the manuscript. SC an RK performed DNA experiments and NGS analysis. PZ and CG performed gene expression analysis. RS, RB performed clinical and physical examinations of the patients. MY, MB, AKAY, and FA performed the audiological evaluation. AH and JNE performed the literature review and helped in writing the manuscript. All authors have read and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent
Approval to conduct the study was obtained from the Institutional Review Board of the Lebanese American University, Beirut, Lebanon (IRB #:LAU.SOM.EC1.2020.R3.1/Feb/2024). All family members signed an informed consent for participation and sample collection.
Consent to publish
All family members signed an informed consent for data publication.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chouery, E., Mehawej, C., Saade, R. et al. POLD3 haploinsufficiency is linked to non-syndromic sensorineural adult-onset progressive hearing and balance impairments. Eur J Hum Genet 33, 121–130 (2025). https://doi.org/10.1038/s41431-024-01715-7
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41431-024-01715-7