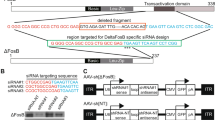
Abstract
L-3,4-dihydroxyphenylalanine (L-DOPA) is currently the preferred treatment for Parkinson’s Disease (PD) and is considered the gold standard. However, prolonged use of L-DOPA in patients can result in involuntary movements known as Levodopa-induced dyskinesia (LID), which includes uncontrollable dystonia affecting the trunk, limbs, and face. The role of ΔFosB protein, a truncated splice variant of the FosB gene, in LID has been acknowledged, but its underlying mechanism has remained elusive. Here, using a mouse model of Parkinson’s disease treated with chronic levodopa we demonstrate that serum response factor (SRF) binds to the FosB promoter, thereby activating FosB expression and levodopa induced-dyskinetic movements. Western blot analysis demonstrates a significant increase in SRF expression in the dyskinetic group compared to the control group. Knocking down SRF significantly reduced abnormal involuntary movements (AIMS) and ΔFosB expression compared to the control. Conversely, overexpression of SRF led to an increase in ΔFosB expression and worsened levodopa-induced dyskinesia. To shed light on the regulatory role of the Akt signaling pathway in this phenomenon, we administered the Akt agonist SC79 to PD mouse models via intraperitoneal injection, followed by L-DOPA administration. The expression of SRF, ΔFosB, and phosphorylated Akt (p-Akt) significantly increased in this group compared to the group receiving normal saline to signify that these happen through Akt signaling pathway. Collectively, our findings identify a promising therapeutic target for addressing levodopa-induced dyskinesia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of this study are available on request from the corresponding authors [PAK & TCX].
References
Tran TN, Vo TNN, Frei K, Truong DD. Levodopa-induced dyskinesia: clinical features, incidence, and risk factors. J Neural Transm (Vienna). 2018;125:1109–17.
di Biase L, Pecoraro PM, Carbone SP, Caminiti ML, Di Lazzaro V. Levodopa-induced dyskinesias in Parkinson’s disease: an overview on pathophysiology, clinical manifestations, therapy management strategies and future directions. J Clin Med. 2023;12:4427.
Xiao B, Tan EK. Cell replacement for Parkinson’s disease: advances and challenges. Neural Regen Res. 2023;18:2693–4.
Bezard E, Brotchie JM, Gross CE. Pathophysiology of levodopa-induced dyskinesia: potential for new therapies. Nat Rev Neurosci. 2001;2:577–88.
Eusebi P, Romoli M, Paoletti FP, Tambasco N, Calabresi P, Parnetti L. Risk factors of levodopa-induced dyskinesia in Parkinson’s disease: results from the PPMI cohort. NPJ Parkinsons Dis. 2018;4:33.
Cao X, Yasuda T, Uthayathas S, Watts RL, Mouradian MM, Mochizuki H, et al. Striatal overexpression of DeltaFosB reproduces chronic levodopa-induced involuntary movements. J Neurosci. 2010;30:7335–43.
Svenningsson P, Arts J, Gunne L, Andren PE. Acute and repeated treatment with L-DOPA increase c-jun expression in the 6-hydroxydopamine-lesioned forebrain of rats and common marmosets. Brain Res. 2002;955:8–15.
Pavon N, Martin AB, Mendialdua A, Moratalla R. ERK phosphorylation and FosB expression are associated with L-DOPA-induced dyskinesia in hemiparkinsonian mice. Biol Psychiatry. 2006;59:64–74.
Palafox-Sanchez V, Sosti V, Ramirez-Garcia G, Kulisevsky J, Aguilera J, Limon ID. Differential expression of striatal DeltaFosB mRNA and FosB mRNA after different levodopa treatment regimens in a rat model of Parkinson’s disease. Neurotox Res. 2019;35:563–74.
Sabatakos G, Rowe GC, Kveiborg M, Wu M, Neff L, Chiusaroli R, et al. Doubly truncated FosB isoform (Delta2DeltaFosB) induces osteosclerosis in transgenic mice and modulates expression and phosphorylation of Smads in osteoblasts independent of intrinsic AP-1 activity. J Bone Min Res. 2008;23:584–95.
Robison AJ, Nestler EJ. DeltaFOSB: a potentially druggable master orchestrator of activity-dependent gene expression. ACS Chem Neurosci. 2022;13:296–307.
Chen J, Kelz MB, Hope BT, Nakabeppu Y, Nestler EJ. Chronic Fos-related antigens: stable variants of deltaFosB induced in brain by chronic treatments. J Neurosci. 1997;17:4933–41.
Nestler EJ. ∆FosB: a transcriptional regulator of stress and antidepressant responses. Eur J Pharm. 2015;753:66–72.
Beck G, Zhang J, Fong K, Mochizuki H, Mouradian MM, Papa SM. Striatal DeltaFosB gene suppression inhibits the development of abnormal involuntary movements induced by L-Dopa in rats. Gene Ther. 2021;28:760–70.
Beck G, Singh A, Zhang J, Potts LF, Woo JM, Park ES, et al. Role of striatal DeltaFosB in l-Dopa-induced dyskinesias of parkinsonian nonhuman primates. Proc Natl Acad Sci USA. 2019;116:18664–72.
Engeln M, Bastide MF, Toulme E, Dehay B, Bourdenx M, Doudnikoff E, et al. Selective inactivation of striatal FosB/DeltaFosB-expressing neurons alleviates L-DOPA-induced dyskinesia. Biol Psychiatry. 2016;79:354–61.
Jenner P. Molecular mechanisms of L-DOPA-induced dyskinesia. Nat Rev Neurosci. 2008;9:665–77.
Kambey PA, Liu WY, Wu J, Bosco B, Nadeem I, Kanwore K, et al. Single-nuclei RNA sequencing uncovers heterogenous transcriptional signatures in Parkinson’s disease associated with nuclear receptor-related factor 1 defect. Neural Regen Res. 2023;18:2037–46.
Kambey PA, Liu WY, Wu J, Tang C, Buberwa W, Saro A, et al. Amphiregulin blockade decreases the levodopa-induced dyskinesia in a 6-hydroxydopamine Parkinson’s disease mouse model. CNS Neurosci Ther. 2023;29:2925–39.
Kambey PA, Chengcheng M, Xiaoxiao G, Abdulrahman AA, Kanwore K, Nadeem I, et al. The orphan nuclear receptor Nurr1 agonist amodiaquine mediates neuroprotective effects in 6-OHDA Parkinson’s disease animal model by enhancing the phosphorylation of P38 mitogen-activated kinase but not PI3K/AKT signaling pathway. Metab Brain Dis. 2021;36:609–25.
Monville C, Torres EM, Dunnett SB. Validation of the l-dopa-induced dyskinesia in the 6-OHDA model and evaluation of the effects of selective dopamine receptor agonists and antagonists. Brain Res Bull. 2005;68:16–23.
Dekundy A, Lundblad M, Danysz W, Cenci MA. Modulation of L-DOPA-induced abnormal involuntary movements by clinically tested compounds: further validation of the rat dyskinesia model. Behav Brain Res. 2007;179:76–89.
Cantuti-Castelvetri I, Hernandez LF, Keller-McGandy CE, Kett LR, Landy A, Hollingsworth ZR, et al. Levodopa-induced dyskinesia is associated with increased thyrotropin releasing hormone in the dorsal striatum of hemi-parkinsonian rats. PLoS One. 2010;5:e13861.
Zhu JL, Wu YY, Wu D, Luo WF, Zhang ZQ, Liu CF. SC79, a novel Akt activator, protects dopaminergic neuronal cells from MPP(+) and rotenone. Mol Cell Biochem. 2019;461:81–9.
Li Y, Feng D, Wang Z, Zhao Y, Sun R, Tian D, et al. Ischemia-induced ACSL4 activation contributes to ferroptosis-mediated tissue injury in intestinal ischemia/reperfusion. Cell Death Differ. 2019;26:2284–99.
Goyal A, Agrawal A, Verma A, Dubey N. The PI3K-AKT pathway: a plausible therapeutic target in Parkinson’s disease. Exp Mol Pathol. 2023;129:104846.
Long HZ, Cheng Y, Zhou ZW, Luo HY, Wen DD, Gao LC. PI3K/AKT signal pathway: a target of natural products in the prevention and treatment of Alzheimer’s disease and Parkinson’s disease. Front Pharm. 2021;12:648636.
Tran J, Anastacio H, Bardy C. Genetic predispositions of Parkinson’s disease revealed in patient-derived brain cells. NPJ Parkinsons Dis. 2020;6:8.
Abbott A. Levodopa: the story so far. Nature. 2010;466:S6–7.
Calne DB, Sandler M. L-Dopa and Parkinsonism. Nature. 1970;226:21–4.
Raket LL, Oudin Astrom D, Norlin JM, Kellerborg K, Martinez-Martin P, Odin P. Impact of age at onset on symptom profiles, treatment characteristics and health-related quality of life in Parkinson’s disease. Sci Rep. 2022;12:526.
Aizman O, Brismar H, Uhlen P, Zettergren E, Levey AI, Forssberg H, et al. Anatomical and physiological evidence for D1 and D2 dopamine receptor colocalization in neostriatal neurons. Nat Neurosci. 2000;3:226–30.
Filipovic M, Ketzef M, Reig R, Aertsen A, Silberberg G, Kumar A. Direct pathway neurons in mouse dorsolateral striatum in vivo receive stronger synaptic input than indirect pathway neurons. J Neurophysiol. 2019;122:2294–303.
Kurushima H, Ohno M, Miura T, Nakamura TY, Horie H, Kadoya T, et al. Selective induction of DeltaFosB in the brain after transient forebrain ischemia accompanied by an increased expression of galectin-1, and the implication of DeltaFosB and galectin-1 in neuroprotection and neurogenesis. Cell Death Differ. 2005;12:1078–96.
Milde-Langosch K. The Fos family of transcription factors and their role in tumourigenesis. Eur J Cancer. 2005;41:2449–61.
Gualdrini F, Esnault C, Horswell S, Stewart A, Matthews N, Treisman R. SRF Co-factors Control the Balance between Cell Proliferation and Contractility. Mol Cell. 2016;64:1048–61.
Onuh JO, Qiu H. Serum response factor-cofactor interactions and their implications in disease. FEBS J. 2021;288:3120–34.
Chai J, Tarnawski AS. Serum response factor: discovery, biochemistry, biological roles and implications for tissue injury healing. J Physiol Pharm. 2002;53:147–57.
Song J, Dikwella N, Sinske D, Roselli F, Knoll B. SRF deletion results in earlier disease onset in a mouse model of amyotrophic lateral sclerosis. JCI Insight. 2023;8:e167694.
Cheng XY, Li SY, Mao CJ, Wang MX, Chen J, Wang F, et al. Serum response factor promotes dopaminergic neuron survival via activation of beclin 1-dependent autophagy. Neuroscience. 2018;371:288–95.
Chow N, Bell RD, Deane R, Streb JW, Chen J, Brooks A, et al. Serum response factor and myocardin mediate arterial hypercontractility and cerebral blood flow dysregulation in Alzheimer’s phenotype. Proc Natl Acad Sci USA. 2007;104:823–8.
Vidal-Sancho L, Fernandez-Garcia S, Soles-Tarres I, Alberch J, Xifro X. Decreased myocyte enhancer factor 2 levels in the hippocampus of Huntington’s disease mice are related to cognitive dysfunction. Mol Neurobiol. 2020;57:4549–62.
Parkitna JR, Bilbao A, Rieker C, Engblom D, Piechota M, Nordheim A, et al. Loss of the serum response factor in the dopamine system leads to hyperactivity. FASEB J. 2010;24:2427–35.
Yang SP, Lo CY, Tseng HM, Chao CC. Knockdown of protein kinase CK2 blocked gene expression mediated by brain-derived neurotrophic factor-induced serum response element. Chin J Physiol. 2019;62:63–9.
Gerosa L, Grillo B, Forastieri C, Longaretti A, Toffolo E, Mallei A, et al. SRF and SRFDelta5 splicing isoform recruit corepressor LSD1/KDM1A modifying structural neuroplasticity and environmental stress response. Mol Neurobiol. 2020;57:393–407.
Westin JE, Vercammen L, Strome EM, Konradi C, Cenci MA. Spatiotemporal pattern of striatal ERK1/2 phosphorylation in a rat model of L-DOPA-induced dyskinesia and the role of dopamine D1 receptors. Biol Psychiatry. 2007;62:800–10.
Santini E, Alcacer C, Cacciatore S, Heiman M, Herve D, Greengard P, et al. L-DOPA activates ERK signaling and phosphorylates histone H3 in the striatonigral medium spiny neurons of hemiparkinsonian mice. J Neurochem. 2009;108:621–33.
Fasano S, Bezard E, D’Antoni A, Francardo V, Indrigo M, Qin L, et al. Inhibition of Ras-guanine nucleotide-releasing factor 1 (Ras-GRF1) signaling in the striatum reverts motor symptoms associated with L-dopa-induced dyskinesia. Proc Natl Acad Sci USA. 2010;107:21824–9.
Madhunapantula SV, Mosca PJ, Robertson GP. The Akt signaling pathway: an emerging therapeutic target in malignant melanoma. Cancer Biol Ther. 2011;12:1032–49.
Huang X, Liu G, Guo J, Su Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int J Biol Sci. 2018;14:1483–96.
Rivera VM, Miranti CK, Misra RP, Ginty DD, Chen RH, Blenis J, et al. A growth factor-induced kinase phosphorylates the serum response factor at a site that regulates its DNA-binding activity. Mol Cell Biol. 1993;13:6260–73.
Small EM, O’Rourke JR, Moresi V, Sutherland LB, McAnally J, Gerard RD, et al. Regulation of PI3-kinase/Akt signaling by muscle-enriched microRNA-486. Proc Natl Acad Sci USA. 2010;107:4218–23.
Poser S, Impey S, Trinh K, Xia Z, Storm DR. SRF-dependent gene expression is required for PI3-kinase-regulated cell proliferation. EMBO J. 2000;19:4955–66.
Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta. 2010;1802:396–405.
Funding
This project was funded by Jiangsu Province Science Foundation for Youths (Grant No. BK20210903).
Author information
Authors and Affiliations
Contributions
PAK and TCX conceived the idea, designed and supervised the study; WJ performed the experiments; LWY, MYS and WB performed data analysis.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Laboratory animal care and use protocols approved by the Xuzhou Medical University’s institutional animal care and use committee. This study exclusively utilized mice, therefore consent to participate is not applicable.
Consent for publication
All authors consent to the publication of this manuscript.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kambey, P.A., Wu, J., Liu, W. et al. Targeting serum response factor (SRF) deactivates ΔFosB and mitigates Levodopa-induced dyskinesia in a mouse model of Parkinson’s disease. Gene Ther 31, 614–624 (2024). https://doi.org/10.1038/s41434-024-00492-8
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41434-024-00492-8