Abstract
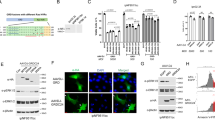
Genetic diseases such as Neurofibromatosis type 1 (NF1) and Charcot-Marie Tooth disease involve Schwann cells (SCs) associated with peripheral nerves. Gene therapy using adeno-associated virus (AAV) vector mediated gene delivery is a promising strategy to treat these diseases. However, AAV-mediated transduction of SCs in vivo after intravascular delivery is relatively inefficient, with a lack of extensive characterization of different capsids to date. Here, we performed an in vivo selection with an AAV9 capsid peptide display library in a mouse model of NF1. We chose one capsid variant, AAV-SC3, which was present in NF1 nerves for comparison to two benchmark capsids after systemic injection. AAV-SC3 significantly outperformed one of the two benchmark capsids at levels of transgene mRNA in the neurofibroma. Immunofluorescence microscopy revealed transgene expressing Sox10-positive SCs throughout the neurofibroma with AAV-SC3 injection. Next, we performed a pooled screen with four of the top capsids from our initial selection and AAV9 and identified one capsid, AAV-SC4, with enhanced biodistribution to and transduction of normal sciatic nerve in mice. This capsid displayed a peptide with a known laminin-binding motif, which may provide a conduit for future laminin-targeting strategies. Our results provide a baseline for future AAV-based gene therapies developed for NF1 or other diseases that affect SCs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Data are available upon request. Plasmid expressing the capsid gene for AAV-SC4 is available at Addgene (Plasmid ID 236254).
Code availability
All code written and used in this study can be accessed by emailing the authors, who will provide scripts and methods of use.
References
Mendell JR, Al-Zaidy SA, Rodino-Klapac LR, Goodspeed K, Gray SJ, Kay CN, et al. Current clinical applications of in vivo gene therapy with AAVs. Mol Ther. 2021;29:464–88.
Keeler AM, Flotte TR. Recombinant adeno-associated virus gene therapy in light of luxturna (and zolgensma and glybera): where are we, and how did we get here? Annu Rev Virol. 2019;6:601–21.
Ozelo MC, Mahlangu J, Pasi KJ, Giermasz A, Leavitt AD, Laffan M, et al. Valoctocogene roxaparvovec gene therapy for hemophilia A. N. Engl J Med. 2022;386:1013–25.
Pipe SW, Leebeek FWG, Recht M, Key NS, Castaman G, Miesbach W, et al. Gene therapy with etranacogene dezaparvovec for hemophilia B. N. Engl J Med. 2023;388:706–18.
Tai CH, Lee NC, Chien YH, Byrne BJ, Muramatsu SI, Tseng SH, et al. Long-term efficacy and safety of eladocagene exuparvovec in patients with AADC deficiency. Mol Ther. 2022;30:509–18.
Mendell JR, Sahenk Z, Lehman K, Nease C, Lowes LP, Miller NF, et al. Assessment of systemic delivery of rAAVrh74.MHCK7.micro-dystrophin in children with duchenne muscular dystrophy: a nonrandomized controlled trial. JAMA Neurol. 2020;77:1122–31.
Kagiava A, Richter J, Tryfonos C, Leal-Julia M, Sargiannidou I, Christodoulou C, et al. Efficacy of AAV serotypes to target Schwann cells after intrathecal and intravenous delivery. Sci Rep. 2021;11:23358.
Kagiava A, Karaiskos C, Richter J, Tryfonos C, Jennings MJ, Heslegrave AJ, et al. AAV9-mediated Schwann cell-targeted gene therapy rescues a model of demyelinating neuropathy. Gene Ther. 2021;28:659–75.
Tanguy Y, Biferi MG, Besse A, Astord S, Cohen-Tannoudji M, Marais T, et al. Systemic AAVrh10 provides higher transgene expression than AAV9 in the brain and the spinal cord of neonatal mice. Front Mol Neurosci. 2015;8:36.
Homs J, Ariza L, Pages G, Udina E, Navarro X, Chillon M, et al. Schwann cell targeting via intrasciatic injection of AAV8 as gene therapy strategy for peripheral nerve regeneration. Gene Ther. 2011;18:622–30.
Hanlon KS, Meltzer JC, Buzhdygan T, Cheng MJ, Sena-Esteves M, Bennett RE, et al. Selection of an Efficient AAV Vector for Robust CNS Transgene Expression. Mol Ther Methods Clin Dev. 2019;15:320–32.
Ortonne N, Carroll SL, Rodriguez FJ, Miller DC, Nazarian RM, Blakeley JO, et al. Assessing interobserver variability and accuracy in the histological diagnosis and classification of cutaneous neurofibromass. Neurooncol Adv. 2020;2:i117–i23.
Zhu Y, Ghosh P, Charnay P, Burns DK, Parada LF. Neurofibromas in NF1: Schwann cell origin and role of tumor environment. Science. 2002;296:920–2.
Woolfenden S, Zhu H, Charest A. A Cre/LoxP conditional luciferase reporter transgenic mouse for bioluminescence monitoring of tumorigenesis. Genesis. 2009;47:659–66.
Prabhakar S, Taherian M, Gianni D, Conlon TJ, Fulci G, Brockmann J, et al. Regression of schwannomas induced by adeno-associated virus-mediated delivery of caspase-1. Hum Gene Ther. 2013;24:152–62.
Ivanchenko MV, Hanlon KS, Devine MK, Tenneson K, Emond F, Lafond JF, et al. Preclinical testing of AAV9-PHP.B for transgene expression in the non-human primate cochlea. Hear Res. 2020;394:107930.
Hanlon KS, Cheng M, Ferrer RM, Ryu JR, Lee B, De La Cruz D, et al. In vivo selection in non-human primates identifies AAV capsids for on-target CSF delivery to spinal cord. Mol Ther. 2024;32:2584–603.
Morrison BM, Tsingalia A, Vidensky S, Lee Y, Jin L, Farah MH, et al. Deficiency in monocarboxylate transporter 1 (MCT1) in mice delays regeneration of peripheral nerves following sciatic nerve crush. Exp Neurol. 2015;263:325–38.
Wu J, Williams JP, Rizvi TA, Kordich JJ, Witte D, Meijer D, et al. Plexiform and dermal neurofibromas and pigmentation are caused by Nf1 loss in desert hedgehog-expressing cells. Cancer Cell. 2008;13:105–16.
Ribeiro S, Napoli I, White IJ, Parrinello S, Flanagan AM, Suter U, et al. Injury signals cooperate with Nf1 loss to relieve the tumor-suppressive environment of adult peripheral nerve. Cell Rep. 2013;5:126–36.
Rizvi TA, Akunuru S, de Courten-Myers G, Switzer RC 3rd, Nordlund ML, et al. Region-specific astrogliosis in brains of mice heterozygous for mutations in the neurofibromatosis type 1 (Nf1) tumor suppressor. Brain Res. 1999;816:111–23.
Hinderer C, Katz N, Buza EL, Dyer C, Goode T, Bell P, et al. Severe toxicity in nonhuman primates and piglets following high-dose intravenous administration of an adeno-associated virus vector expressing human SMN. Hum Gene Ther. 2018;29:285–98.
Huang Q, Chen AT, Chan KY, Sorensen H, Barry AJ, Azari B, et al. Targeting AAV vectors to the central nervous system by engineering capsid-receptor interactions that enable crossing of the blood-brain barrier. PLoS Biol. 2023;21:e3002112.
Kawabata H, Konno A, Matsuzaki Y, Sato Y, Kawachi M, Aoki R, et al. Improving cell-specific recombination using AAV vectors in the murine CNS by capsid and expression cassette optimization. Mol Ther Methods Clin Dev. 2024;32:101185.
Beharry A, Gong Y, Kim JC, Hanlon KS, Nammour J, Hieber K, et al. The AAV9 variant capsid AAV-F mediates widespread transgene expression in nonhuman primate spinal cord after intrathecal administration. Hum Gene Ther. 2022;33:61–75.
Huang Q, Chen AT, Chan KY, Sorensen H, Barry AJ, Azari B, et al. Targeting AAV vectors to the CNS via <em>de novo</em> engineered capsid-receptor interactions. bioRxiv. 2022:2022.10.31.514553.
Akalu A, Roth JM, Caunt M, Policarpio D, Liebes L, Brooks PC. Inhibition of angiogenesis and tumor metastasis by targeting a matrix immobilized cryptic extracellular matrix epitope in laminin. Cancer Res. 2007;67:4353–63.
Jiang M, Chen M, Liu N. Interactions between Schwann cell and extracellular matrix in peripheral nerve regeneration. Front Neurol. 2024;15:1372168.
Dodd RD, Mito JK, Eward WC, Chitalia R, Sachdeva M, Ma Y, et al. NF1 deletion generates multiple subtypes of soft-tissue sarcoma that respond to MEK inhibition. Mol Cancer Ther. 2013;12:1906–17.
Stemmer-Rachamimov AO, Louis DN, Nielsen GP, Antonescu CR, Borowsky AD, Bronson RT, et al. Comparative pathology of nerve sheath tumors in mouse models and humans. Cancer Res. 2004;64:3718–24.
Kershner LJ, Choi K, Wu J, Zhang X, Perrino M, Salomonis N, et al. Multiple Nf1 Schwann cell populations reprogram the plexiform neurofibroma tumor microenvironment. JCI Insight. 2022;7:e154513.
Bohlen MO, McCown TJ, Powell SK, El-Nahal HG, Daw T, Basso MA, et al. Adeno-associated virus capsid-promoter interactions in the brain translate from rat to the nonhuman primate. Hum Gene Ther. 2020;31:1155–68.
Powell SK, Samulski RJ, McCown TJ. AAV capsid-promoter interactions determine CNS cell-selective gene expression in vivo. Mol Ther. 2020;28:1373–80.
Gonzalez-Sandoval A, Pekrun K, Tsuji S, Zhang F, Hung KL, Chang HY, et al. The AAV capsid can influence the epigenetic marking of rAAV delivered episomal genomes in a species dependent manner. Nat Commun. 2023;14:2448.
Okada Y, Hosoi N, Matsuzaki Y, Fukai Y, Hiraga A, Nakai J, et al. Development of microglia-targeting adeno-associated viral vectors as tools to study microglial behavior in vivo. Commun Biol. 2022;5:1224.
McKee KK, Yang DH, Patel R, Chen ZL, Strickland S, Takagi J, et al. Schwann cell myelination requires integration of laminin activities. J Cell Sci. 2012;125:4609–19.
Skoumal M, Seidlits S, Shin S, Shea L. Localized lentivirus delivery via peptide interactions. Biotechnol Bioeng. 2016;113:2033–40.
Chan KY, Jang MJ, Yoo BB, Greenbaum A, Ravi N, Wu WL, et al. Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat Neurosci. 2017;20:1172–9.
Drouyer M, Chu TH, Labit E, Haase F, Navarro RG, Nazareth D, et al. Novel AAV variants with improved tropism for human Schwann cells. Mol Ther Methods Clin Dev. 2024;32:101234.
Zincarelli C, Soltys S, Rengo G, Rabinowitz JE. Analysis of AAV serotypes 1-9 mediated gene expression and tropism in mice after systemic injection. Mol Ther. 2008;16:1073–80.
Romo CG, Piotrowski AF, Campian JL, Diarte J, Rodriguez FJ, Bale TA, et al. Clinical, histological and molecular features of gliomas in adults with neurofibromatosis type 1. Neuro Oncol. 2023;25:1474–86.
Wilson JM, Flotte TR. Moving Forward After Two Deaths in a Gene Therapy Trial of Myotubular Myopathy. Hum Gene Ther. 2020;31:695–6.
Hordeaux J, Lamontagne RJ, Song C, Buchlis G, Dyer C, Buza EL, et al. High-dose systemic adeno-associated virus vector administration causes liver and sinusoidal endothelial cell injury. Mol Ther. 2024;32:952–68.
Xie J, Xie Q, Zhang H, Ameres SL, Hung JH, Su Q, et al. MicroRNA-regulated, systemically delivered rAAV9: a step closer to CNS-restricted transgene expression. Mol Ther. 2011;19:526–35.
Krolak T, Chan KY, Kaplan L, Huang Q, Wu J, Zheng Q, et al. A high-efficiency AAV for endothelial cell transduction throughout the central nervous system. Nat Cardiovasc Res. 2022;1:389–400.
Acknowledgements
We thank Dr. Akiko Yoshinaga for help with genotyping of the neurofibroma model and professional insights into NF1, Ms Suzanne McDavitt for skilled editorial assistance and Ms Diane M. Nguyen for assistance with figure artwork. We thank Dr. Judith S. Kempfle for the recommendation of the anti-Sox10 antibody for the immunofluorescence experiment. We thank Dr. Nancy Ratner for providing Nf1flox/flox mice and helpful review of the manuscript. We thank Dr. K.A. Kleopa for helpful discussion around the timing of the in-life mouse AAV gene transfer experiments.
Funding
This work was supported by NIH R01 grant DC017117 (C.A.M.), the Gilbert Family Foundation Award #521015 (C.A.M.) and the Gilbert Family Foundation Award #521013 (X.O.B.). I.C.H is supported in part by the National Institute of Biomedical Imaging and Bioengineering under award number 1K25EB032864-01A1.
Author information
Authors and Affiliations
Contributions
C.A.M., K.S.H. and X.O.B. conceived of the study, analyzed data, and wrote the manuscript. C.A.M. performed all of the tail vein injections of mice and assisted in tissue harvesting. S.P., P.S.C., C.C.D.H. and E.A.H. maintained the mice colony, breeding, genotyping, sciatic nerve injections, perfusion, and sacrifice of the mice for further analysis. A.S.R. carried out the pathological analyses in collaboration with S.P. and E.A.H. E.A.H. performed the RT-ddPCR analysis on neurofibroma and confocal microscopy. P.E. performed confocal microscopy and image analysis on the neurofibroma from mice injected with the different AAV vectors. N.P. cloned the AAV-P0-tdTomato construct. D.D.L.C. and A.V.C. produced, purified, and titered all AAV vectors used in the study, C.N. and G.W.R. performed cryosectioning of nerves and the immunofluorescence staining of neurofibroma sections. A.C. provided the luciferase floxed mouse model and advised on the study. M.C. assisted with image analysis. I.C.H, S.M. and N.J. performed microscopy analyses. All authors helped edit, revise, and approve of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
CAM has a financial interest in Sphere Gene Therapeutics, Inc., Chameleon Biosciences, Inc., and Skylark Bio, Inc., companies developing gene therapy platforms. CAM’s interests were reviewed and are managed by MGH and Mass General Brigham in accordance with their conflict-of-interest policies.
Ethical approval
All animal procedures were performed in accordance with Massachusetts General Hospital’s (MGH’s) recommendations for the care and use of animals and were maintained and handled under protocols approved by the Institutional Animal Care and Use Committee (IACUC).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abou Haidar, E., Prabhakar, S., Cheah, P.S. et al. Engineered AAV capsids mediate transduction of murine neurofibroma and sciatic nerve. Gene Ther 32, 385–397 (2025). https://doi.org/10.1038/s41434-025-00542-9
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41434-025-00542-9