Abstract
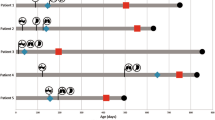
Onasemnogene abeparvovec (OA) is the first gene replacement therapy (GT) approved for 5q spinal muscular atrophy (SMA). While effective, it can cause severe side effects, including thrombotic microangiopathy (TMA). The pathophysiology, risk factors, and management of viral-vector-related TMA remain unclear. This study aimed to evaluate TMA frequency among Brazilian patients treated with OA and characterize their clinical and laboratory profiles. This retrospective, multicenter study analyzed 294 Brazilian patients with 5q SMA treated with OA between October 2020 and September 2024, of whom seven (2.4%) developed TMA. The average age at OA administration was 20.4 months, and the average weight was 11.5 kg. Three patients had documented infections before OA administration. TMA symptoms appeared within 6–10 days post-infusion. All patients showed hemolytic anemia, thrombocytopenia, and at least one organ dysfunction. Treatment included plasmapheresis in two cases and increased corticosteroid doses in four cases. One patient died from TMA complications. Whole exome sequencing in five patients identified no pathogenic variants linked to TMA. TMA is a rare but severe complication of OA therapy for SMA. Prompt recognition and management, often with corticosteroids, are crucial for improving outcomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Data availability
Additional data are available from the corresponding author on reasonable request.
References
Lefebvre S, Burglen L, Reboullet S, Clermont O, Burlet P, Viollet L, et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80:155–65.
Finkel RS, McDermott MP, Kaufmann P, Darras BT, Chung WK, Sproule DM, et al. Observational study of spinal muscular atrophy type I and implications for clinical trials. Neurology. 2014;83:810–7.
Kolb SJ, Coffey CS, Yankey JW, Krosschell K, Arnold WD, Rutkove SB, et al. Natural history of infantile-onset spinal muscular atrophy. Ann Neurol. 2017;82:883–91.
Sugarman EA, Nagan N, Zhu H, Akmaev VR, Zhou Z, Rohlfs EM, et al. Pan-ethnic carrier screening and prenatal diagnosis for spinal muscular atrophy: clinical laboratory analysis of >72,400 specimens. Eur J Hum Genet 2012;20:27–32.
Zanoteli E, Araujo A, Becker MM, Fortes C, Franca MC Jr, et al. Consensus from the Brazilian Academy of Neurology for the diagnosis, genetic counseling, and use of disease-modifying therapies in 5q spinal muscular atrophy. Arq Neuropsiquiatr. 2024;82:1–18.
Blair HA. Onasemnogene Abeparvovec: A Review in Spinal Muscular Atrophy. CNS Drugs. 2022;36:995–1005.
Mendell JR, Al-Zaidy S, Shell R, Arnold WD, Rodino-Klapac LR, Prior TW, et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N Engl J Med. 2017;377:1713–22.
Gowda V, Atherton M, Murugan A, Servais L, Sheehan J, Standing E, et al. Efficacy and safety of onasemnogene abeparvovec in children with spinal muscular atrophy type 1: real-world evidence from 6 infusion centres in the United Kingdom. Lancet Reg Health Eur. 2024;37:100817.
Yang D, Ruan Y, Chen Y. Safety and efficacy of gene therapy with onasemnogene abeparvovec in the treatment of spinal muscular atrophy: A systematic review and meta-analysis. J Paediatr Child Health. 2023;59:431–8.
Chand DH, Zaidman C, Arya K, Millner R, Farrar MA, Mackie FE, et al. Thrombotic microangiopathy following onasemnogene abeparvovec for spinal muscular atrophy: a case series. J Pediatr. 2021;231:265–8.
Feldman AG, Parsons JA, Dutmer CM, Veerapandiyan A, Hafberg E, Maloney N, et al. Subacute liver failure following gene replacement therapy for spinal muscular atrophy type 1. J Pediatr. 2020;225:252–8.e1.
Guillou J, de Pellegars A, Porcheret F, Fremeaux-Bacchi V, Allain-Launay E, Debord C, et al. Fatal thrombotic microangiopathy case following adeno-associated viral SMN gene therapy. Blood Adv. 2022;6:4266–70.
Mendonça RH, Ortega AB, Matsui C Jr, van der Linden V, Kerstenetzky M, et al. Gene replacement therapy for spinal muscular atrophy: safety and preliminary efficacy in a Brazilian cohort. Gene Ther. 2024;31:391–9.
Day JW, Mendell JR, Mercuri E, Finkel RS, Strauss KA, Kleyn A, et al. Clinical trial and postmarketing safety of onasemnogene abeparvovec therapy. Drug Saf. 2021;44:1109–19.
Urra M, Lyons S, Teodosiu CG, Burwick R, Java A. Thrombotic microangiopathy in pregnancy: current understanding and management strategies. Kidney Int Rep. 2024;9:2353–71.
Schwotzer N, El Sissy C, Desguerre I, Fremeaux-Bacchi V, Servais L, Fakhouri F. Thrombotic microangiopathy as an emerging complication of viral vector-based gene therapy. Kidney Int Rep. 2024;9:1995–2005.
Al-Nouri ZL, Reese JA, Terrell DR, Vesely SK, George JN. Drug-induced thrombotic microangiopathy: a systematic review of published reports. Blood. 2015;125:616–8.
Palma LMP, Sridharan M, Sethi S. Complement in secondary thrombotic microangiopathy. Kidney Int Rep. 2021;6:11–23.
Scully M, Cataland S, Coppo P, de la Rubia J, Friedman KD, Kremer Hovinga J, et al. Consensus on the standardization of terminology in thrombotic thrombocytopenic purpura and related thrombotic microangiopathies. J Thromb Haemost. 2017;15:312–22.
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–24.
Mazzierli T, Allegretta F, Maffini E, Allinovi M. Drug-induced thrombotic microangiopathy: an updated review of causative drugs, pathophysiology, and management. Front Pharm. 2022;13:1088031.
Palma LMP, Vaisbich-Guimaraes MH, Sridharan M, Tran CL, Sethi S. Thrombotic microangiopathy in children. Pediatr Nephrol. 2022;37:1967–80.
Tsai HM. The molecular biology of thrombotic microangiopathy. Kidney Int. 2006;70:16–23.
Zheng XL, Sadler JE. Pathogenesis of thrombotic microangiopathies. Annu Rev Pathol. 2008;3:249–77.
Weiss C, Becker LL, Friese J, Blaschek A, Hahn A, Illsinger S, et al. Efficacy and safety of gene therapy with onasemnogene abeparvovec in children with spinal muscular atrophy in the D-A-CH-region: a population-based observational study. Lancet Reg Health Eur. 2024;47:101092.
Ruggiero R, Balzano N, Nicoletti MM, di Mauro G, Fraenza F, Campitiello MR, et al. Real-world safety data of the orphan drug onasemnogene abeparvovec (Zolgensma((R))) for the SMA rare disease: a pharmacovigilance study based on the EMA adverse event reporting system. Pharmaceuticals (Basel). 2024;17:394
Waldrop MA, Chagat S, Storey M, Meyer A, Iammarino M, Reash N, et al. Continued safety and long-term effectiveness of onasemnogene abeparvovec in Ohio. Neuromuscul Disord. 2024;34:41–8.
Yazaki K, Sakuma S, Hikita N, Fujimaru R, Hamazaki T. Child neurology: pathologically confirmed thrombotic microangiopathy caused by onasemnogene abeparvovec treatment for SMA. Neurology. 2022;98:808–13.
Day JW, Finkel RS, Chiriboga CA, Connolly AM, Crawford TO, Darras BT, et al. Onasemnogene abeparvovec gene therapy for symptomatic infantile-onset spinal muscular atrophy in patients with two copies of SMN2 (STR1VE): an open-label, single-arm, multicentre, phase 3 trial. Lancet Neurol. 2021;20:284–93.
Mercuri E, Muntoni F, Baranello G, Masson R, Boespflug-Tanguy O, Bruno C, et al. Onasemnogene abeparvovec gene therapy for symptomatic infantile-onset spinal muscular atrophy type 1 (STR1VE-EU): an open-label, single-arm, multicentre, phase 3 trial. Lancet Neurol. 2021;20:832–41.
Aigner C, Gaggl M, Kain R, Prohaszka Z, Garam N, Csuka D, et al. Sex differences in clinical presentation and outcomes among patients with complement-gene-variant-mediated thrombotic microangiopathy. J Clin Med. 2020;9:964
Desguerre I, Barrois R, Audic F, Barnerias C, Chabrol B, Davion JB, et al. Real-world multidisciplinary outcomes of onasemnogene abeparvovec monotherapy in patients with spinal muscular atrophy type 1: experience of the French cohort in the first three years of treatment. Orphanet J Rare Dis. 2024;19:344.
Servais L, Day JW, De Vivo DC, Kirschner J, Mercuri E, et al. Real-World Outcomes in Patients with Spinal Muscular Atrophy Treated with Onasemnogene Abeparvovec Monotherapy: Findings from the RESTORE Registry. J Neuromuscul Dis. 2024;11:425–42.
Othman M, Labelle A, Mazzetti I, Elbatarny HS, Lillicrap D. Adenovirus-induced thrombocytopenia: the role of von Willebrand factor and P-selectin in mediating accelerated platelet clearance. Blood. 2007;109:2832–9.
Salabarria SM, Corti M, Coleman KE, Wichman MB, Berthy JA, D’Souza P, et al. Thrombotic microangiopathy following systemic AAV administration is dependent on anti-capsid antibodies. J Clin Invest. 2024;134.
Tian J, Xu Z, Smith JS, Hofherr SE, Barry MA, Byrnes AP. Adenovirus activates complement by distinctly different mechanisms in vitro and in vivo: indirect complement activation by virions in vivo. J Virol. 2009;83:5648–58.
Witte D, Hartmann H, Drube J, Haffner D, Illsinger S. [Thrombotic microangiopathy (TMA) after gene replacemant therapy (GRT) due to spinal muscular atrophy: case summary and recommendations for treatment]. Klin Padiatr. 2022;234:42–7.
Servais L, Horton R, Saade D, Bonnemann C, Muntoni F. st Ewsg. 261st ENMC International Workshop: management of safety issues arising following AAV gene therapy. 17th-19th June 2022, Hoofddorp, The Netherlands. Neuromuscul Disord. 2023;33:884–96.
Greenberg B, Taylor M, Adler E, Colan S, Ricks D, Yarabe P, et al. Phase 1 study of AAV9.LAMP2B gene therapy in Danon disease. N Engl J Med. 2025;392:972–83.
Duan D. Lethal immunotoxicity in high-dose systemic AAV therapy. Mol Ther. 2023;31:3123–6.
Laforet GA. Thrombotic microangiopathy associated with systemic adeno-associated virus gene transfer: review of reported cases. Hum Gene Ther. 2025;36:64–76.
Acknowledgements
We would like to thank the researchers, physicians and patients for taking part in this study and providing the information included.
Funding
It was not obtained any funding agreements with agencies that may be interested in publishing this article.
Author information
Authors and Affiliations
Contributions
CGC and EZ: Study concept, acquisition and analysis of data, literature review, and initial draft of the paper. RHM: Study concept, acquisition, and analysis of data. CAMM, SMCM and AZ: Analysis of data, and critical revision of manuscript for intellectual content. JC, ABO, CCDL, RdSMC, HVDL, NSCdC, JG-G and JMC: acquisition and analysis of data.
Corresponding author
Ethics declarations
Competing interests
The authors declare that participated in the development and realization of the article. The authors agree with the time limit for publication. From this date, it becomes public responsibility for its contents. It was not obtained any funding agreements with agencies that may be interested in publishing this article. The article has not been and will not be submitted to other printed or electronic journals. We are also aware that all articles accepted for publication must be published with DOI (digital object identifier). The authors state that there was no conflict of interest during the performance and publication of this article.
Ethical approval
The study received approval from the local ethics committee (63660222.5.0000.0068 CAPPESQ), and written informed consent was obtained from the patients’ parents. All methods were performed in accordance with the relevant guidelines and regulations.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Camelo, C.G., Mendonça, R.H., Moreno, C.A.M. et al. Thrombotic microangiopathy following gene therapy for 5q-spinal muscular atrophy. Gene Ther 32, 594–598 (2025). https://doi.org/10.1038/s41434-025-00545-6
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41434-025-00545-6