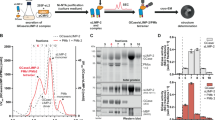
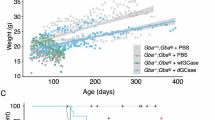
Abstract
Gaucher disease (GD) is a rare genetically inherited illness caused by loss of lysosomal acid β-glucosidase (β-GCase) that leads to progressive accumulation of substrates, sphingolipid glucosylceramide (GL1) and glucosylsphingosine (lyso-GL1). The protein-based enzyme replacement therapy (ERT) requires frequent dosing due to short drug half-life causing challenges in long-term patient compliance. JCXH-301 is a lipid nanoparticle (LNP) encapsulated messenger RNA (mRNA) encoding β-GCase. Intravenous administration of JCXH-301 delivered the target mRNA to various tissues in mice with intracellular expression of β-GCase predominantly in macrophages and dendritic cells in the spleen and bone marrow. In GBA1 D427V homozygous mice treated with JCXH-301, the dose-dependent in vivo production of functional β-GCase resulted in reduction of serum lyso-GL1, a key biomarker of GD. The therapeutic effect of JCXH-301 was sustained for a duration significantly longer than that of protein-based ERT Cerezyme. JCXH-301 administration induced minimal pro-inflammatory cytokines in the liver and spleen. Taken together, these results provide proof-of-concept for using LNP-delivered mRNA as a new drug modality to restore the β-GCase genetic deficiency for GD treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The sequences generated in this study are available in the NCBI database (Accession Gene ID. 2629 and 14466). The other data related to this study are available from the corresponding author upon request, with the permission of Immorna Biotechnology/Immorna Biotherapeutics.
References
Sheth J, Bhavsar R, Mistri M, Pancholi D, Bavdekar A, Dalal A, et al. Gaucher disease: single gene molecular characterization of one-hundred Indian patients reveals novel variants and the most prevalent mutation. BMC Med Genet. 2019;20:31.
Stirnemann J, Belmatoug N, Camou F, Serratrice C, Froissart R, Caillaud C, et al. A review of gaucher disease pathophysiology, clinical presentation and treatments. Int J Mol Sci. 2017;18:441.
Vardi A, Zigdon H, Meshcheriakova A, Klein AD, Yaacobi C, Eilam R, et al. Delineating pathological pathways in a chemically induced mouse model of Gaucher disease. J Pathol. 2016;239:496–509.
Hruska KS, LaMarca ME, Scott CR, Sidransky E. Gaucher disease: mutation and polymorphism spectrum in the glucocerebrosidase gene (GBA). Hum Mutat. 2008;29:567–83.
Cox TM, Cachon-Gonzalez MB. The cellular pathology of lysosomal diseases. J Pathol. 2012;226:241–54.
Panicker LM, Miller D, Awad O, Bose V, Lun Y, Park TS, et al. Gaucher iPSC-derived macrophages produce elevated levels of inflammatory mediators and serve as a new platform for therapeutic development. Stem Cells. 2014;32:2338–49.
Kishnani PS, Al-Hertani W, Balwani M, Goker-Alpan O, Lau HA, Wasserstein M, et al. Screening, patient identification, evaluation, and treatment in patients with Gaucher disease: Results from a Delphi consensus. Mol Genet Metab. 2022;135:154–62.
Weinreb NJ. Imiglucerase and its use for the treatment of Gaucher’s disease. Expert Opin Pharmacother. 2008;9:1987–2000.
Murugesan V, Chuang W-L, Liu J, Lischuk A, Kacena K, Lin H, et al. Glucosylsphingosine is a key biomarker of Gaucher disease. Am J Hematol. 2016;91:1082–9.
Gary SE, Ryan E, Steward AM, Sidransky E. Recent advances in the diagnosis and management of Gaucher disease. Expert Rev Endocrinol Metab. 2018;13:107–18.
Yap TL, Velayati A, Sidransky E, Lee JC. Membrane-bound alpha-synuclein interacts with glucocerebrosidase and inhibits enzyme activity. Mol Genet Metab. 2013;108:56–64.
Badieyan ZS, Evans T. Concise review: application of chemically modified mRNA in cell fate conversion and tissue engineering. Stem Cells Transl Med. 2019;8:833–43.
Nelson J, Sorensen EW, Mintri S, Rabideau AE, Zheng W, Besin G, et al. Impact of mRNA chemistry and manufacturing process on innate immune activation. Sci Adv. 2020;6:eaaz6893.
Vaidyanathan S, Azizian KT, Haque A, Henderson JM, Hendel A, Shore S, et al. Uridine depletion and chemical modification increase Cas9 mRNA activity and reduce immunogenicity without HPLC purification. Mol Ther Nucleic Acids. 2018;12:530–42.
Xu S, Yang K, Li R, Zhang L. mRNA vaccine era-mechanisms, drug platform and clinical prospection. Int J Mol Sci. 2020;21:6582.
Martini PGV, Guey LT. A new era for rare genetic diseases: messenger RNA therapy. Hum Gene Ther. 2019;30:1180–9.
Bernal JA. RNA-based tools for nuclear reprogramming and lineage-conversion: towards clinical applications. J Cardiovasc Transl Res. 2013;6:956–68.
Berraondo P, Martini PGV, Avila MA, Fontanellas A. Messenger RNA therapy for rare genetic metabolic diseases. Gut. 2019;68:1323–30.
An D, Frassetto A, Jacquinet E, Eybye M, Milano J, DeAntonis C, et al. Long-term efficacy and safety of mRNA therapy in two murine models of methylmalonic acidemia. EBioMedicine. 2019;45:519–28.
Dekker N, van Dussen L, Hollak CEM, Overkleeft H, Scheij S, Ghauharali K, et al. Elevated plasma glucosylsphingosine in Gaucher disease: relation to phenotype, storage cell markers, and therapeutic response. Blood. 2011;118:e118–e27.
Polinski NK, Martinez TN, Gorodinsky A, Gareus R, Sasner M, Herberth M, et al. Decreased glucocerebrosidase activity and substrate accumulation of glycosphingolipids in a novel GBA1 D409V knock-in mouse model. PLOS ONE. 2021;16:e0252325.
Sardi SP, Clarke J, Kinnecom C, Tamsett TJ, Li L, Stanek LM, et al. CNS expression of glucocerebrosidase corrects -synuclein pathology and memory in a mouse model of Gaucher-related synucleinopathy. Proc Natl Acad Sci USA. 2011;108:12101–6.
Xu YH, Quinn B, Witte D, Grabowski GA. Viable mouse models of acid beta-glucosidase deficiency: the defect in Gaucher disease. Am J Pathol. 2003;163:2093–101.
Xu Y-H, Sun Y, Barnes S, Grabowski GA, Schiffmann R. Comparative therapeutic effects of velaglucerase alfa and imiglucerase in a Gaucher disease mouse model. PloS ONE. 2010;5:e10750.
Zheng W, Du S, Tian M, Xu W, Tian Y, Li T, et al. Lepidium meyenii Walp exhibits anti-inflammatory activity against ConA-induced acute hepatitis. Mediat Inflamm. 2018;2018:8982756.
Ji YR, Kim HJ, Bae KB, Lee S, Kim MO, Ryoo ZY. Hepatic serum amyloid A1 aggravates T cell-mediated hepatitis by inducing chemokines via Toll-like receptor 2 in mice. J Biol Chem. 2015;290:12804–11.
de Fost M, Aerts JM, Hollak CE. Gaucher disease: from fundamental research to effective therapeutic interventions. Neth J Med. 2003;61:3–8.
Campbell TN, Choy FY. Knockdown of chimeric glucocerebrosidase by green fluorescent protein-directed small interfering RNA. Genet Mol Res. 2004;3:282–7.
Cox TM. Gaucher disease: clinical profile and therapeutic developments. Biologics. 2010;4:299–313.
Kido J, Sugawara K, Nakamura K. Gene therapy for lysosomal storage diseases: current clinical trial prospects. Front Genet. 2023;14:1064924.
Hughes DA, Ferrante F. GALILEO-1: a phase I/II safety and efficacy study of FLT201 gene therapy for Gaucher Disease Type 1. Future Rare Dis. 2023;3:FRD35.
Rastall DP, Amalfitano A. Recent advances in gene therapy for lysosomal storage disorders. Appl Clin Genet. 2015;8:157–69.
Sevin C, Deiva K. Clinical trials for gene therapy in lysosomal diseases with CNS involvement. Front Mol Biosci. 2021;8:624988.
Du S, Ou H, Cui R, Jiang N, Zhang M, Li X, et al. Delivery of glucosylceramidase beta gene using AAV9 vector therapy as a treatment strategy in mouse models of gaucher disease. Hum Gene Ther. 2019;30:155–67.
Tucci F, Scaramuzza S, Aiuti A, Mortellaro A. Update on clinical ex vivo hematopoietic stem cell gene therapy for inherited monogenic diseases. Mol Ther. 2021;29:489–504.
Hudry E, Vandenberghe LH. Therapeutic AAV gene transfer to the nervous system: a clinical reality. Neuron. 2019;102:263.
Dahl M, Smith EMK, Warsi S, Rothe M, Ferraz MJ, Aerts J, et al. Correction of pathology in mice displaying Gaucher disease type 1 by a clinically-applicable lentiviral vector. Mol Ther Methods Clin Dev. 2021;20:312–23.
Corbau R, Miranda C, Comper F, Kalcheva P, Chisari E, Cocita C, et al. FLT201: an AAV-mediated gene therapy for type 1 Gaucher disease designed to target difficult to reach tissues. Mol Genet Metab. 2021;132:S28–S9.
Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7:27–31.
Koeberl D, Schulze A, Sondheimer N, Lipshutz GS, Geberhiwot T, Li L, et al. Interim analyses of a first-in-human phase 1/2 mRNA trial for propionic acidaemia. Nature. 2024;628:872–7.
Cohn DM, Gurugama P, Magerl M, Katelaris CH, Launay D, Bouillet L, et al. CRISPR-based therapy for hereditary angioedema. N. Engl J Med. 2025;392:458–67.
Pharmacoeconomic Review Report: Eliglustat (Cerdelga): (Sanofi Genzyme): Indication: Gaucher Disease Type 1 [Internet]. CADTH Common Drug Reviews. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2017.
Acknowledgements
We thank Shanghai Model Organisms Center Inc. and Ms. Dongli Liang of Shanghai Jiaotong University for their expert assistance in mouse experiments. We thank Mr. Ji Xu and Ms. Yanqi Su for the logistical support.
Funding
The study was fully funded by Immorna Biotechnology/Immorna Biotherapeutics.
Author information
Authors and Affiliations
Contributions
Z.W., Z.G., S.W., Y.C., and Y.L. developed the concept and designed the studies. S.W. and Z.Z performed the experiments and curated the data. S.W., Z.Z., and Y.C. analyzed the data. S.W., Y.C., X.Q., and Y.L. wrote the manuscript. All the authors reviewed the manuscript and agreed this version to be published.
Corresponding author
Ethics declarations
Competing interests
All authors are employees of Immorna Biotechnology/Immorna Biotherapeutics. Z.W., Y. L., and Z. G. are inventors to the Chinese patent CN118903476B.
Ethics approval statement
All animal experiments were performed in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals and relevant Chinese laws and regulations. All animal use adhered to the IACUC guidelines for animal ethics and welfare. The in vivo experiment protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of Shanghai Jiaotong University, China (authorization number: A2022044), and Shanghai Model Organisms Center, Inc., China (authorization number: No. 2020-0047).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g., a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Y., Wang, S., Chen, Y. et al. Systemically delivered lipid nanoparticle-mRNA encoding lysosomal acid β-glucosidase restores the enzyme deficiency in a murine Gaucher disease model. Gene Ther (2025). https://doi.org/10.1038/s41434-025-00549-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41434-025-00549-2