Abstract
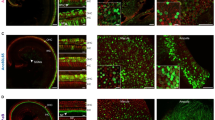
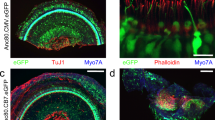
Vesicular glutamate transporter 3 (VGLUT3) is prominently expressed in the inner hair cells of the cochlea, playing a vital role in auditory signal transmission to the brain. Previous studies have shown that Vglut3 gene knockout in mice causes severe sensorineural hearing loss without affecting hair cell integrity. However, the cochlear structure of the aged Vglut3KO remains inadequately explored. In this study, we analyzed the cochlear structure of aged Vglut3KO mice, revealing significant degeneration of inner hair cells, synapses, and stereocilia. To explore the potential of gene therapy to restore cochlear structure, we employed AAV8 vectors to express Vglut3 in the cochleae of 5-week-old Vglut3KO mice. Twenty-seven weeks post-injection, we conducted a series of experiments to evaluate the efficacy of our gene therapy approach. Auditory brainstem response (ABR) testing demonstrated restoration of auditory function following gene therapy. Immunohistochemical staining and scanning electron microscopy (SEM) analysis revealed substantial recovery of inner hair cells and stereocilia post-injection. Our findings provide important insights into the development of novel therapeutic strategies for age-related hearing loss.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data are available from the corresponding author upon reasonable request.
References
Xue Y, Tao Y, Wang X, Wang X, Shu Y, Liu Y, et al. RNA base editing therapy cures hearing loss induced by OTOF gene mutation. Mol Ther. 2023;31:3520–30.
Lin FR, Pike JR, Albert MS, Arnold M, Burgard S, Chisolm T, et al. Hearing intervention versus health education control to reduce cognitive decline in older adults with hearing loss in the USA (ACHIEVE): a multicentre, randomised controlled trial. Lancet. 2023;402:786–97.
de Rijk SR, Boys AJ, Roberts IV, Jiang C, Garcia C, Owens RM, et al. Tissue-engineered cochlear fibrosis model links complex impedance to fibrosis formation for cochlear implant patients. Adv Healthc Mater. 2023;12:e2300732.
Ma CC, Wang ZL, Xu T, He ZY, Wei YQ. The approved gene therapy drugs worldwide: from 1998 to 2019. Biotechnol Adv. 2020;40:107502.
Petit C, Bonnet C, Safieddine S. Deafness: from genetic architecture to gene therapy. Nat Rev Genet. 2023;24:665–86.
Akil O, Seal RP, Burke K, Wang C, Alemi A, During M, et al. Restoration of hearing in the VGLUT3 knockout mouse using virally mediated gene therapy. Neuron. 2012;75:283–93.
Gao X, Tao Y, Lamas V, Huang M, Yeh WH, Pan B, et al. Treatment of autosomal dominant hearing loss by in vivo delivery of genome editing agents. Nature. 2018;553:217–21.
Lv J, Wang H, Cheng X, Chen Y, Wang D, Zhang L, et al. AAV1-hOTOF gene therapy for autosomal recessive deafness 9: a single-arm trial. Lancet. 2024;403:2317–25.
Qi J, Tan F, Zhang L, Lu L, Zhang S, Zhai Y, et al. AAV-mediated gene therapy restores hearing in patients with DFNB9 deafness. Adv Sci. 2024;11:e2306788.
Wang H, Chen Y, Lv J, Cheng X, Cao Q, Wang D, et al. Bilateral gene therapy in children with autosomal recessive deafness 9: single-arm trial results. Nat Med. 2024;30:1898–904.
Akil O, Dyka F, Calvet C, Emptoz A, Lahlou G, Nouaille S, et al. Dual AAV-mediated gene therapy restores hearing in a DFNB9 mouse model. Proc Natl Acad Sci USA. 2019;116:4496–501.
Al-Moyed H, Cepeda AP, Jung S, Moser T, Kügler S, Reisinger E. A dual-AAV approach restores fast exocytosis and partially rescues auditory function in deaf otoferlin knock-out mice. EMBO Mol Med. 2019;11:e9396.
Jiang L, Wang D, He Y, Shu Y. Advances in gene therapy hold promise for treating hereditary hearing loss. Mol Ther. 2023;31:934–50.
Zhao X, Jin C, Dong T, Sun Z, Zheng X, Feng B, et al. Characterization of promoters for adeno-associated virus mediated efficient Cas9 activation in adult Cas9 knock-in murine cochleae. Hearing Res. 2020;394:107999.
Zhao X, Liu H, Liu H, Cai R, Wu H. Gene therapy restores auditory functions in an adult Vglut3 knockout mouse model. Hum Gene Ther. 2022;33:729–39.
Kang W, Zhao X, Sun Z, Dong T, Jin C, Tong L, et al. Adeno-associated virus vector enables safe and efficient Cas9 activation in neonatal and adult Cas9 knockin murine cochleae. Gene Ther. 2020;27:392–405.
Bi Z, Li X, Ren M, Gu Y, Zhu T, Li S, et al. Development and transdifferentiation into inner hair cells require Tbx2. Natl Sci Rev. 2022;9:nwac156.
Ruel J, Emery S, Nouvian R, Bersot T, Amilhon B, Van Rybroek JM, et al. Impairment of SLC17A8 encoding vesicular glutamate transporter-3, VGLUT3, underlies nonsyndromic deafness DFNA25 and inner hair cell dysfunction in null mice. Am J Hum Genet. 2008;83:278–92.
Seal RP, Akil O, Yi E, Weber CM, Grant L, Yoo J, et al. Sensorineural deafness and seizures in mice lacking vesicular glutamate transporter 3. Neuron. 2008;57:263–75.
Mathiesen BK, Miyakoshi LM, Cederroth CR, Tserga E, Versteegh C, Bork PAR, et al. Delivery of gene therapy through a cerebrospinal fluid conduit to rescue hearing in adult mice. Sci Transl Med. 2023;15:eabq3916.
Tao Y, Huang M, Shu Y, Ruprecht A, Wang H, Tang Y, et al. Delivery of adeno-associated virus vectors in adult mammalian inner-ear cell subtypes without auditory dysfunction. Hum Gene Ther. 2018;29:492–506.
Stalmann U, Franke AJ, Al-Moyed H, Strenzke N, Reisinger E. Otoferlin is required for proper synapse maturation and for maintenance of inner and outer hair cells in mouse models for DFNB9. Front Cell Neurosci. 2021;15:677543.
Qi J, Zhang L, Tan F, Zhang Y, Zhou Y, Zhang Z, et al. Preclinical efficacy and safety evaluation of AAV-OTOF in DFNB9 mouse model and nonhuman primate. Adv Sci. 2024;11:e2306201.
Tan F, Chu C, Qi J, Li W, You D, Li K, et al. AAV-ie enables safe and efficient gene transfer to inner ear cells. Nat Commun. 2019;10:3733.
Qi J, Tan F, Zhang L, Zhou Y, Zhang Z, Sun Q, et al. Critical role of TPRN rings in the stereocilia for hearing. Mol Ther. 2024;32:204–17.
Zheng Z, Li G, Cui C, Wang F, Wang X, Xu Z, et al. Preventing autosomal-dominant hearing loss in Bth mice with CRISPR/CasRx-based RNA editing. Signal Transduct Target Ther. 2022;7:79.
Liu L, Zou L, Li K, Hou H, Hu Q, Liu S, et al. Template-independent genome editing in the Pcdh15(av-3j) mouse, a model of human DFNB23 nonsyndromic deafness. Cell Rep. 2022;40:111061.
Shubina-Oleinik O, Nist-Lund C, French C, Rockowitz S, Shearer AE, Holt JR. Dual-vector gene therapy restores cochlear amplification and auditory sensitivity in a mouse model of DFNB16 hearing loss. Sci Adv. 2021;7:eabi7629.
Xue Y, Hu X, Wang D, Li D, Li Y, Wang F, et al. Gene editing in a Myo6 semi-dominant mouse model rescues auditory function. Mol Ther. 2022;30:105–18.
Gu X, Hu X, Wang D, Xu Z, Wang F, Li D, et al. Treatment of autosomal recessive hearing loss via in vivo CRISPR/Cas9-mediated optimized homology-directed repair in mice. Cell Res. 2022;32:699–702.
Xiao Q, Xu Z, Xue Y, Xu C, Han L, Liu Y, et al. Rescue of autosomal dominant hearing loss by in vivo delivery of mini dCas13X-derived RNA base editor. Sci Transl Med. 2022;14:eabn0449.
Yu Q, Liu S, Guo R, Chen K, Li Y, Jiang D, et al. Complete restoration of hearing loss and cochlear synaptopathy via minimally invasive, single-dose, and controllable middle ear delivery of brain-derived neurotrophic factor-poly(dl-lactic acid-co-glycolic acid)-loaded hydrogel. ACS Nano. 2024;18:5995–6730.
Tao Y, Lamas V, Du W, Zhu W, Li Y, Whittaker MN, et al. Treatment of monogenic and digenic dominant genetic hearing loss by CRISPR-Cas9 ribonucleoprotein delivery in vivo. Nat Commun. 2023;14:4928.
Tao Y, Liu X, Yang L, Chu C, Tan F, Yu Z, et al. AAV-ie-K558R mediated cochlear gene therapy and hair cell regeneration. Signal Transduct Target Ther. 2022;7:109.
Liu H, Liu H, Wang L, Song L, Jiang G, Lu Q, et al. Cochlear transcript diversity and its role in auditory functions implied by an otoferlin short isoform. Nat Commun. 2023;14:3085.
Moser T, Starr A. Auditory neuropathy–neural and synaptic mechanisms. Nat Rev Neurol. 2016;12:135–49.
Moser T, Grabner CP, Schmitz F. Sensory processing at ribbon synapses in the retina and the cochlea. Physiol Rev. 2020;100:103–44.
Obholzer N, Wolfson S, Trapani JG, Mo W, Nechiporuk A, Busch-Nentwich E, et al. Vesicular glutamate transporter 3 is required for synaptic transmission in zebrafish hair cells. J Neurosci. 2008;28:2110–8.
El Mestikawy S, Wallén-Mackenzie A, Fortin GM, Descarries L, Trudeau LE. From glutamate co-release to vesicular synergy: vesicular glutamate transporters. Nat Rev Neurosci. 2011;12:204–16.
Wu Z, Grillet N, Zhao B, Cunningham C, Harkins-Perry S, Coste B, et al. Mechanosensory hair cells express two molecularly distinct mechanotransduction channels. Nat Neurosci. 2017;20:24–33.
Liu H, Giffen KP, Chen L, Henderson HJ, Cao TA, Kozeny GA, et al. Molecular and cytological profiling of biological aging of mouse cochlear inner and outer hair cells. Cell Rep. 2022;39:110665.
Lek A, Wong B, Keeler A, Blackwood M, Ma K, Huang S, et al. Death after high-dose rAAV9 gene therapy in a patient with Duchenne’s muscular dystrophy. N Engl J Med. 2023;389:1203–10.
Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, Dolan DF, et al. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med. 2005;11:271–6.
Acknowledgements
This work was supported by the China Postdoctoral Science Foundation (2024M752977), the Medical Science and Technology Research Plan Joint Construction Project of Henan Province (LHGJ20240279), the National Natural Science Foundation of China (82371160), and the Postdoctoral Research Foundation of the First Affiliated Hospital of Zhengzhou University (72137). We also thank the Precision Medicine Center, Academy of Medical Sciences, Zhengzhou University, and the Key Laboratory for Research on Deafness Mechanism, Henan Provincial Health Commission (Henan Health Science and Education Letter 2021, No. 44) for their support.
Author information
Authors and Affiliations
Contributions
LW initiated and managed the project, while LW and WT conceived and designed the experiments. XZ, HX, and CL performed most of the experiments and data analysis. All authors contributed to data analysis, interpretation, and presentation. XZ and HX wrote the manuscript with contributions from all authors. The supplementary experiments to answer the reviewer’s questions were mainly completed by WL, XZ and RZ.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All methods were performed in accordance with the relevant guidelines and regulations. All animals were bred in accredited barrier facilities with a controlled environment, feed, and water. The mice were housed in a room with a 12-h light/12-h dark cycle, and both water and food were provided ad libitum. All relevant research was approved by the Institutional Animal Care and Use Committee of Zhengzhou University (Ethics ID: ZZU-LAC20230526 (08)). All experiments were conducted in accordance with the 3Rs principles of animal research: Reduction, Replacement, and Refinement. Animals exhibiting severe health impairment were systematically excluded from analysis based on the following objective indicators: weight loss exceeding 20% of baseline body weight. Informed consent for publication was obtained from all participants.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, X., Xu, H., Lian, C. et al. Gene therapy restores auditory function and rescues damaged inner hair cells in an aged Vglut3 knockout mouse model. Gene Ther (2025). https://doi.org/10.1038/s41434-025-00558-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41434-025-00558-1