Abstract
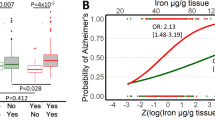
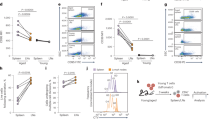
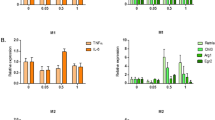
Apolipoprotein E (ApoE) plays a crucial role in iron homeostasis in the body, while macrophages are the principal cells responsible for handling iron in mammals. However, it is unknown whether ApoE can affect the functional subtypes and the iron handling capacity of splenic macrophages (SM). Here, we investigated the effects of ApoE deficiency (ApoE−/−) on the polarization and iron content of SM and its potential mechanisms. ApoE−/− was found to induce a significant increase in the expressions of M1 marker genes CD86, IL-1β, IL-6, IL-12, TNF-α and iNOS and a reduction in M2 marker genes CD206, Arg-1, IL-10 and Ym-1 in SM of mice aged 28 weeks, Meanwhile, ApoE−/− caused a significant increase in iron content and expression of ferritin, transferrin receptor 1 (TfR1), iron regulatory protein 1 (IRP1) and heme oxygenase-1 (HO-1) and a reduction in ferroportin1 (Fpn1) in spleen and/or SM of mice aged 28 weeks. It was concluded that ApoE−/− can increase iron content through increased iron uptake mediated by TfR/ IRPs and decreased iron release mediated by Fpn1, leading to polarization of the SM to M1 phenotype.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 digital issues and online access to articles
$119.00 per year
only $19.83 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
References
Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer’s disease. Neuron. 2009;63:287303.
Ayton S, Faux NG, Bush AI. Alzheimer’s Disease Neuroimaging Initiative. Ferritin levels in the cerebrospinal fluid predict Alzheimer’s disease outcomes and are regulated by APOE. Nat Commun. 2015;6:6760.
Mahoney-Sanchez L, Belaidi AA, Bush AI, Ayton S. The complex role of apolipoprotein E in Alzheimer’s disease: an overview and update. J Mol Neurosci. 2016;60:325–35.
Guerreiro R, Ross OA, Kun-Rodrigues C, Hernandez DG, Orme T, Eicher JD, et al. Investigating the genetic architecture of dementia with Lewy bodies: a two-stage genome-wide association study. Lancet Neurol. 2018;17:64–74.
Lee TS, Shiao MS, Pan CC, Chau LY. Iron-deficient diet reduces atherosclerotic lesions in apoE-deficient mice. Circulation. 1999;99:1222–9.
Meir KS, Leitersdorf E. Atherosclerosis in the apolipoprotein-E-deficient mouse: a decade of progress. Arterioscler Thromb Vasc Biol. 2004;24:1006–14.
Yu T, Parks BW, Yu S, Srivastava R, Gupta K, Wu X, et al. Iron-ion radiation accelerates atherosclerosis in apolipoprotein E-deficient mice. Radiat Res. 2011;175:766–73.
Ma J, Ma HM, Shen MQ, Wang YY, Bao YX, Liu Y, et al. The role of iron in atherosclerosis in apolipoprotein E deficient mice. Front Cardiovasc Med. 2022;9:857933.
Guo Q, Qian C, Qian ZM. Iron metabolism and atherosclerosis. Trends Endocrinol Metab. 2023;34:404–13.
Xu H, Perreau VM, Dent KA, Bush AI, Finkelstein DI, Adlard PA. Iron regulates apolipoprotein E expression and secretion in neurons and astrocytes. J Alzheimers Dis. 2016;51:471–87.
Britton LJ, Bridle K, Jaskowski LA, He J, Ng C, Ruelcke JE, et al. Iron inhibits the secretion of apolipoproteine in cultured human adipocytes. Cell Mol Gastroenterol Hepatol. 2018;6:215–e8.
Lynch JR, Morgan D, Mance J, Matthew WD, Laskowitz DT. Apolipoprotein E modulates glial activation and the endogenous central nervous system inflammatory response. J Neuroimmunol. 2001;114:107–13.
Frühbeck G. The adipose tissue as a source of vasoactive factors. Curr Med Chem Cardiovasc Hematol Agents. 2004;2:197–208.
Zhu Y, Kodvawala A, Hui DY. Apolipoprotein E inhibits toll-like receptor (TLR)-3- and TLR-4-mediated macrophage activation through distinct mechanisms. Biochem J. 2010;428:47–54.
Wrighting DM, Andrews NC. Interleukin-6 induces hepcidin expression through STAT3. Blood. 2006;108:3204–9.
Verga Falzacappa MV, Vujic Spasic M, Kessler R, Stolte J, Hentze MW, Muckenthaler MU. STAT3 mediates hepatic hepcidin expression and its inflammatory stimulation. Blood. 2007;109:353–8.
Qian ZM, He X, Liang T, Wu KC, Yan YC, Lu LN, et al. Lipopolysaccharides upregulate hepcidin in neuron via microglia and the IL-6/STAT3 signaling pathway. Mol Neurobiol. 2014;50:811–20.
Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–55.
Yang L, Yang Y, Chen Y, Xu Y, Peng J. Cell-based drug delivery systems and their in vivo fate. Adv Drug Deliv Rev. 2022;187:114394.
Cesta MF. Normal structure, function, and histology of the spleen. Toxicol Pathol. 2006;34:455–65.
Marro S, Chiabrando D, Messana E, Stolte J, Turco E, Tolosano E, et al. Heme controls ferroportin1 (FPN1) transcription involving Bach1, Nrf2 and a MARE/ARE sequence motif at position -7007 of the FPN1 promoter. Haematologica. 2010;95:1261–8.
Korolnek T, Hamza I. Macrophages and iron trafficking at the birth and death of red cells. Blood. 2015;125:2893–7.
Soares MP, Hamza I. Macrophages and iron metabolism. Immunity. 2016;44:492–504.
Winn NC, Volk KM, Hasty AH. Regulation of tissue iron homeostasis: the macrophage “ferrostat. JCI Insight. 2020;5:e132964.
A-Gonzalez N, Castrillo A. Origin and specialization of splenic macrophages. Cell Immunol. 2018;330:151–8.
Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Ann Rev Immunol. 2009;27:451–83.
Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–96.
Biswas SK, Mantovani A. Orchestration of metabolism by macrophages. Cell Metab. 2012;15:432–7.
Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233:6425–40.
Ma J, Qian C, Bao Y, Liu MY, Ma HM, Shen MQ, et al. Apolipoprotein E deficiency induces a progressive increase in tissue iron contents with age in mice. Redox Biol. 2021;40:101865.
Ma J, Guo Q, Shen MQ, Li W, Zhong QX, Qian ZM. Apolipoprotein E is required for brain iron homeostasis in mice. Redox Biol. 2023;64:102779.
Qian ZM, Xiao DS, Ke Y, Liao QK. Increased nitric oxide is one of the causes of changes of iron metabolism in strenuously exercised rats. Am J Physiol Regul Integr Comp Physiol. 2001;280:R739–43.
Xu GJ, Hannappel E, Morgan J, Hempstead J, Horecker BL. Splenic macrophages were isolated from mouse spleen according to synthesis of thymosin beta 4 by peritoneal macrophages and adherent spleen cells. Proc Natl Acad Sci USA. 1982;79:4006–9.
Chang YZ, Ke Y, Du JR, Halpern GM, Ho KP, Zhu L, et al. Increased divalent metal transporter 1 expression might be associated with the neurotoxicity of L-DOPA. Mol Pharm. 2006;69(3):968–74.
Du F, Qian ZM, Gong Q, Zhu ZJ, Lu L, Ke Y. The iron regulatory hormone hepcidin inhibits the expression of iron release as well as iron uptake proteins in J774 cells. J Nutr Biochem. 2012;23:1694–700.
Qian ZM, Chang YZ, Leung G, Du JR, Zhu L, Wang Q, et al. Expression of ferroportin1, hephaestin and ceruloplasmin in rat heart. Biochim Biophys Acta. 2007;1772:527–32.
Zhou YF, Zhang C, Yang G, Qian ZM, Zhang MW, Ma J, et al. Hepcidin protects neuron from hemin-mediated injury by reducing iron. Front Physiol. 2017;8:332.
Hentze MW, Muckenthaler MU, Andrews NC. Balancing acts: molecular control of mammalian iron metabolism. Cell. 2004;117:285–97.
Galy B, Ferring-Appel D, Kaden S, Gröne HJ, Hentze MW. Iron regulatory proteins are essential for intestinal function and control key iron absorption molecules in the duodenum. Cell Metab. 2008;7:79–85.
Muckenthaler MU, Galy B, Hentze MW. Systemic iron homeostasis and the iron-responsive element/iron-regulatory protein (IRE/IRP) regulatory network. Annu Rev Nutr. 2008;28:97–213.
Du F, Qian C, Qian ZM, Wu XM, Xie H, Yung WH, et al. Hepcidin directly inhibits transferrin receptor 1 expression in astrocytes via a cyclic AMP-protein kinase A pathway. Glia. 2011;59:936–45.
Du F, Qian ZM, Luo Q, Yung WH, Ke Y. Hepcidin suppresses brain iron accumulation by downregulating iron transport proteins in iron-overloaded rats. Mol Neurobiol. 2015;52:101–14.
Kanzaki H, Shinohara F, Kanako I, Yamaguchi Y, Fukaya S, Miyamoto Y, et al. Molecular regulatory mechanisms of osteoclastogenesis through cytoprotective enzymes. Redox Biol. 2016;8:186–91.
Soares MP, Bach FH. Heme oxygenase-1: from biology to therapeutic potential. Trends Mol Med. 2009;15(2):50–8.
Okabe Y, Medzhitov R. Tissue biology perspective on macrophages. Nat Immunol. 2016;17:9–17.
Schultze JL, Schmieder A, Goerdt S. Macrophage activation in human diseases. Semin Immunol. 2015;27:249–56.
Zhou YF, Wu XM, Zhou G, Mu MD, Zhang FL, Li FM, et al. Cystathionine β-synthase is required for body iron homeostasis. Hepatology. 2018;67:21–35.
Tanno T, Bhanu NV, Oneal PA, Goh SH, Staker P, Lee YT, et al. High levels of GDF15 in thalassemia suppress expression of the iron regulatory protein hepcidin. Nat Med. 2007;13:1096–101.
Hentze MW, Muckenthaler MU, Galy B, Camaschella C. Two to tango: regulation of Mammalian iron metabolism. Cell. 2010;142:24–38.
Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–3.
Nairz M, Schleicher U, Schroll A, Sonnweber T, Theurl I, Ludwiczek S, et al. Nitric oxide-mediated regulation of ferroportin-1 controls macrophage iron homeostasis and immune function in Salmonella infection. Exp Med. 2013;210:855–73.
Gozzelino R, Soares MP. Coupling heme and iron metabolism via ferritin H chain. Antioxid Redox Signal. 2014;20:1754–69.
Harrison PM, Arosio P. The ferritins: molecular properties, iron storage function and cellular regulation. Biochim Biophys Acta. 1996;275:61–203.
Knutson MD, Oukka M, Koss LM, Aydemir F, Wessling-Resnick M. Iron release from macrophages after erythrophagocytosis is up-regulated by ferroportin 1 overexpression and down-regulated by hepcidin. Proc Natl Acad Sci USA. 2005;102:1324–8.
Alam MZ, Devalaraja S, Haldar M. The heme connection: linking erythrocytes and macrophage biology. Front Immunol. 2017;8:33.
Bellomo A, Gentek R, Golub R, Bajénoff M. Macrophage-fibroblast circuits in the spleen. Immunol Rev. 2021;302:104–25.
Murray PJ. Macrophage polarization. Annu Rev Physiol. 2017;79:541–66.
Corna G, Campana L, Pignatti E, Castiglioni A, Tagliafico E, Bosurgi L, et al. Polarization dictates iron handling by inflammatory and alternatively activated macrophages. Haematologica. 2010;95:1814–22.
Recalcati S, Locati M, Marini A, Santambrogio P, Zaninotto F, De Pizzol M, et al. Differential regulation of iron homeostasis during human macrophage polarized activation. Eur J Immunol. 2010;40:824–35.
Agoro R, Mura C. Inflammation-induced up-regulation of hepcidin and down-regulation of ferroportin transcription are dependent on macrophage polarization. Blood Cells Mol Dis. 2016;61:16–25.
Cairo G, Recalcati S, Mantovani A, Locati M. Iron trafficking and metabolism in macrophages: contribution to the polarized phenotype. Trends Immunol. 2011;32:241–7.
Acknowledgements
We would like to thank Christopher Qian of the Chinese University of Hong Kong for assisting with the preparation and English revision of this manuscript.
Funding
The studies in our laboratories were supported by the National Natural Science Foundation of China (82003702, 31571195).
Author information
Authors and Affiliations
Contributions
Meng-Qi Shen: Conceptualization, Formal analysis, Methodology, Writing—original draft. Qian Guo: Conceptualization, Methodology, Supervision, Writing—review & editing. Wei Li: Conceptualization, Formal analysis, Methodology, Visualization, Writing—original draft. Zhong-Ming Qian: Conceptualization, Methodology, Supervision, Writing—review & editing. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All animal care and experimental protocols in this study were performed according to the Animal Management Rules of the Ministry of Health of China, and approved by the Animal Ethics Committees of Nantong University (NSFC31571195). All mice were sacrificed at 28 weeks old for the designed measurements.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shen, MQ., Guo, Q., Li, W. et al. Apolipoprotein E deficiency leads to the polarization of splenic macrophages towards M1 phenotype by increasing iron content. Genes Immun 25, 381–388 (2024). https://doi.org/10.1038/s41435-024-00290-7
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41435-024-00290-7
This article is cited by
-
Infiltrating macrophages replace Kupffer cells and play diverse roles in severe alcohol-associated hepatitis
Cellular & Molecular Immunology (2025)