Abstract
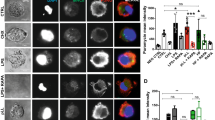
Dendritic cells (DCs) are professional antigen presentation cells (APCs) that bridge innate and adaptive immune functions to contain pathogenic threats. Long noncoding RNAs (lncRNAs) are implicated in regulating biological processes, including inflammation and immunity. However, the knowledge of myeloid DC-expressed lncRNA repertoire and their regulatory functions is limited. Here we profiled lncRNA expression kinetics during monocyte-to-DC (moDC) differentiation and characterized their functional roles. Our RNA-seq data identified a repertoire of differentially expressed lncRNAs associated with moDC differentiation and a large subset of these lncRNAs are distinct from M1 or M2 macrophages. We selected two DC-enriched lncRNAs and observed that PARAL1 silencing, or overexpression modulates DC surface markers expression. Importantly, PARAL1 RNAi significantly reduced, while its overexpression upregulated the levels of multiple TLRs. Upon treatment with TLR agonists PARAL1 knockdown cells exhibit reduced NF-κB, IRF3 and IRF7 phosphorylation substantiating its role in potentiating TLR signaling. Mechanistically, PARAL1 silencing showed significant downregulation of multiple NF-κB-induced genes and time-dependent inhibition of proinflammatory cytokine secretion upon challenge with TLR agonists. Finally, PARAL1 RNAi in DCs significantly impaired antigen processing and presentation to T cells. Overall, this study characterized novel functions of PARAL1 in regulating DC differentiation, TLR-dependent innate immunity and activation of adaptive immune response.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 digital issues and online access to articles
$119.00 per year
only $19.83 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The raw RNA sequencing data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database under the accession number GSE285316 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE285316). All study data are included in the article and Supplementary files.
References
Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–61.
Liu J, Zhang X, Cheng Y, Cao X. Dendritic cell migration in inflammation and immunity. Cell Mol Immunol. 2021;18:2461–71.
Ulvmar MH, Werth K, Braun A, Kelay P, Hub E, Eller K, et al. The atypical chemokine receptor CCRL1 shapes functional CCL21 gradients in lymph nodes. Nat Immunol. 2014;15:623–30.
Braun A, Worbs T, Moschovakis GL, Halle S, Hoffmann K, Bölter J, et al. Afferent lymph-derived T cells and DCs use different chemokine receptor CCR7-dependent routes for entry into the lymph node and intranodal migration. Nat Immunol. 2011;12:879–87.
Ahmad I, Valverde A, Ahmad F, Naqvi AR. Long Noncoding RNA in Myeloid and Lymphoid Cell Differentiation, Polarization and Function. Cells 2020;9:269.
Bocchetti M, Scrima M, Melisi F, Luce A, Sperlongano R, Caraglia M, et al. LncRNAs and Immunity: Coding the Immune System with Noncoding Oligonucleotides. Int J Mol Sci. 2021;22:1741.
Mattick JS, Amaral PP, Carninci P, Carpenter S, Chang HY, Chen LL, et al. Long non-coding RNAs: definitions, functions, challenges and recommendations. Nat Rev Mol Cell Biol. 2023;24:430–47.
Ahmad I, Valverde A, Naqvi RA, Naqvi AR. Long Non-coding RNAs RN7SK and GAS5 Regulate Macrophage Polarization and Innate Immune Responses. Front Immunol. 2020;11:604981.
Wang P, Xue Y, Han Y, Lin L, Wu C, Xu S, et al. The STAT3-binding long noncoding RNA lnc-DC controls human dendritic cell differentiation. Science 2014;344:310–13.
Zhou Y, Chen X, Zheng Y, Shen R, Sun S, Yang F, et al. Long non-coding RNAs and mRNAs expression profiles of monocyte-derived dendritic cells from PBMCs in AR. Front Cell Dev Biol. 2021;9:636477.
Xin J, Li J, Feng Y, Wang L, Zhang Y, Yang R. Downregulation of long noncoding RNA HOTAIRM1 promotes monocyte/dendritic cell differentiation through competitively binding to endogenous miR-3960. Onco Targets Ther. 2017;10:1307–15.
Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell 2010;140:805–20.
Kawasaki T, Kawai T. Toll-like receptor signaling pathways. Front Immunol. 2014;5:461.
Zhao Q, Pang G, Yang L, Chen S, Xu R, Shao W. Long Noncoding RNAs Regulate the Inflammatory Responses of Macrophages. Cells 2021;11:5.
Carpenter S, Aiello D, Atianand MK, Ricci EP, Gandhi P, Hall LL, et al. A long noncoding RNA mediates both activation and repression of immune response genes. Science 2013;341:789–92.
Li Z, Chao TC, Chang KY, Lin N, Patil VS, Shimizu C, et al. The long noncoding RNA THRIL regulates TNFα expression through its interaction with hnRNPL. Proc Natl Acad Sci USA. 2014;111:1002–7.
Böyum A. solation of leucocytes from human blood. Further observations. Methylcellulose, dextran, and ficoll as erythrocyte aggregating agents. Scand J Clin Lab Invest Suppl. 1968;97:31–50.
Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011;17:10–2.
Langmead B, Trapnell C, Pop M, Salzberg SL. Salzberg Ultrafast and memory- efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10:R25.
Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14:R36.
Pertea M, Kim D, Pertea GM, Leek JT, Salzberg SL. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat Protoc. 2016;11:1650–67.
Naqvi AR, Fordham JB, Nares S. miR-24, miR-30b, and miR-142-3p regulate phagocytosis in myeloid inflammatory cells. J Immunol. 2015;194:1916–27.
Kong L, Zhang Y, Ye ZQ, Liu XQ, Zhao SQ, Wei L, et al. CPC: assess the protein-coding potential of transcripts using sequence features and support vector machine. Nucl Acid Res. 2007;35:W345–9.
Guo JC, Fang SS, Wu Y, Zhang JH, Chen Y, Liu J, et al. CNIT: a fast and accurate web tool for identifying protein-coding and long non-coding transcripts based on intrinsic sequence composition. Nucleic Acids Res. 2019;47:W516–W522.
Guilliams M, Ginhoux F, Jakubzick C, Naik SH, Onai N, Schraml BU, et al. Dendritic Cells, Monocytes and Macrophages: A Unified Nomenclature Based on Ontogeny. Nat Rev Immunol. 2014;14:571–78.
Bocchetti M, Scrima M, Melisi F, Luce A, Sperlongano R, Caraglia M, et al. LncRNAs and Immunity: Coding the Immune System with Noncoding Oligonucleotides. Int J Mol Sci. 2021;9:1741.
Fordham JB, Naqvi AR, Nares S. Regulation of miR-24, miR-30b, and miR-142-3p during macrophage and dendritic cell differentiation potentiates innate immunity. J Leukoc Biol. 2015;98:195–207.
Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–84.
Kaisho T, Akira S. Regulation of dendritic cell function through Toll-like receptors. Curr Mol Med. 2003;4:373–85.
Jarrossay D, Napolitani G, Colonna M, Sallusto F, Lanzavecchia A. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. European J Immunol. 2001;11:3388–93.
Muralidharan S, Mandrekar P. Cellular stress response and innate immune signaling: integrating pathways in host defense and inflammation. J Leukoc Biol. 2013;94:1167–84.
Bernard D, Prasanth KV, Tripathi V, Colasse S, Nakamura T, Xuan Z, et al. A long nuclear-retained noncoding RNA regulates synaptogenesis by modulating gene expression. EMBO J. 2010;29:3082–93.
Kryger R, Fan L, Wilce PA, Jaquet V. MALAT-1, a non protein-coding RNA is upregulated in the cerebellum, hippocampus and brain stem of human alcoholics. Alcohol 2012;46:629–34.
Fang R, Wang C, Jiang Q, Lv M, Gao P, Yu X, et al. NEMO-IKKβ Are Essential for IRF3 and NF-κB Activation in the cGAS-STING Pathway. J Immunol. 2017;199:3222–33.
Hemmi H, Akira S. TLR signaling and the function of dendritic cells. Chem Immunol Allergy. 2005;86:120–35.
Fitzgerald KA, Rowe DC, Barnes BJ, Caffrey DR, Visintin A, Latz E, et al. LPS-TLR4 signaling to IRF-3/7 and NF- kappaB involves the toll adapters TRAM and TRIF. J Exp Med. 2003;198:1043–55.
Doyle S, Vaidya S, O’Connell R, Dadgostar H, Dempsey P, Wu T, et al. IRF3 mediates a TLR3/TLR4-specific antiviral gene program. Immunity 2002;17:251–63.
Ning S, Pagano JS, Barber GN. IRF7: activation, regulation, modification, and function. Genes Immun. 2011;12:399–414.
Xing Y, Cao R, Hu HM. TLR and NLRP3 inflammasome-dependent innate immune responses to tumor-derived autophagosomes (DRibbles). Cell Death Dis. 2016;7:e2322.
Marchese FP, Raimondi I, Huarte M. The multidimensional mechanisms of long noncoding RNA function. Genome Biol. 2017;18:206.
Dhanoa JK, Sethi RS, Verma R, Arora JS, Mukhopadhyay CS. Long non-coding RNA: Its evolutionary relics and biological implications in mammals: A review. J Anim Sci Technol. 2018;60:25.
Salviano-Silva A, Lobo-Alves SC, Almeida RC, Malheiros D, Petzl-Erler ML. Besides Pathology: Long Non-Coding RNA in Cell and Tissue Homeostasis. Non-Coding. RNA 2018;4:3.
Lee KMC, Achuthan AA, Hamilton JA. GM-CSF: A Promising Target in Inflammation and Autoimmunity. Immunotargets Ther. 2020;9:225–40.
Li Z, Zhang Q, Wu Y, Hu F, Gu L, Chen T, et al. lncRNA Malat1 modulates the maturation process, cytokine secretion and apoptosis in airway epithelial cell-conditioned dendritic cells. Exp Ther Med. 2018;16:3951–58.
Zhang M, Zheng Y, Sun Y, Li S, Chen L, Jin X, et al. Knockdown of NEAT1 induces tolerogenic phenotype in dendritic cells by inhibiting activation of NLRP3 inflammasome. Theranostics 2019;9:3425–42.
Wang Y, Chen S, Chen S, Du J, Lin J, Qin H, et al. Long noncoding RNA expression profile and association with SLEDAI score in monocyte-derived dendritic cells from patients with systematic lupus erythematosus. Arthritis Res Ther. 2018;20:138.
Li Q, Li P, Su J, Liu S, Yang X, Yang Y, et al. LncRNA NKILA was upregulated in diabetic cardiomyopathy with early prediction values. Exp Ther Med. 2019;18:1221–25.
Li H, Zhang GX, Chen Y, Xu H, Fitzgerald DC, Zhao Z, et al. CD11c+CD11b+ dendritic cells play an important role in intravenous tolerance and the suppression of experimental autoimmune encephalomyelitis. J Immunol. 2008;181:2483–93.
Swiecki M, Colonna M. The multifaceted biology of plasmacytoid dendritic cells. Nat Rev Immunol. 2015;15:471–85.
Swiecki M, Gilfillan S, Vermi W, Wang Y, Colonna M. Plasmacytoid dendritic cell ablation impacts early interferon responses and antiviral NK and CD8(+) T cell accrual. Immunity 2010;33:955–66.
Rowland SL, Riggs JM, Gilfillan S, Bugatti M, Vermi W, Kolbeck R, et al. Early, transient depletion of plasmacytoid dendritic cells ameliorates autoimmunity in a lupus model. J Exp Med. 2014;211:1977–91.
Sisirak V, Ganguly D, Lewis KL, Couillault C, Tanaka L, Bolland S, et al. Genetic evidence for the role of plasmacytoid dendritic cells in systemic lupus erythematosus. J Exp Med. 2014;211:1969–76.
Ah Kioon MD, Tripodo C, Fernandez D, Kirou KA, Spiera RF, Crow MK, et al. Plasmacytoid dendritic cells promote systemic sclerosis with a key role for TLR8. Sci Transl Med. 2018;10:eaam8458.
Brewitz A, Eickhoff S, Dähling S, Quast T, Bedoui S, Kroczek RA, et al. CD8(+) T cells orchestrate pDC-XCR1(+) dendritic cell spatial and functional cooperativity to optimize priming. Immunity 2017;46:205–19.
Schlitzer A, McGovern N, Teo P, Zelante T, Atarashi K, Low D, et al. IRF4 transcription factor-dependent CD11b+ dendritic cells in human and mouse control mucosal IL-17 cytokine responses. Immunity 2013;38:970–83.
Williams JW, Tjota MY, Clay BS, Vander Lugt B, Bandukwala HS, Hrusch CL, et al. Transcription factor IRF4 drives dendritic cells to promote Th2 differentiation. Nat Commun. 2013;4:2990.
Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, et al. Differential antigen processing by dendritic cell subsets in vivo. Science 2007;315:107–11.
Saito Y, Komori S, Kotani T, Murata Y, Matozaki T. The Role of Type-2 Conventional Dendritic Cells in the Regulation of Tumor Immunity. Cancers. 2022;14:1976.
Duan T, Du Y, Xing C, Wang HY, Wang RF. Toll-Like Receptor Signaling and Its Role in Cell-Mediated Immunity. Front Immunol. 2022;13:812774.
Cui H, Xie N, Tan Z, Banerjee S, Thannickal VJ, Abraham E, et al. The human long noncoding RNA lnc-IL7R regulates the inflammatory response. Eur J Immunol. 2014;44:2085–95.
Saha S, Murthy S, Rangarajan PN. Identification and characterization of a virus‐inducible non‐coding RNA in mouse brain. J Gen Virol. 2006;87:1991–95.
Imamura K, Imamachi N, Akizuki G, Kumakura M, Kawaguchi A, Nagata K, et al. Long noncoding RNA NEAT1‐dependent SFPQ relocation from promoter region to paraspeckle mediates IL8 expression upon immune stimuli. Mol Cell. 2014;53:393–06.
Li H, Hao Y, Zhang D, Fu R, Liu W, Zhang X, et al. Aberrant expression of long noncoding RNA TMEVPG1 in patients with primary immune thrombocytopenia. Autoimmunity. 2016;49:496–502.
Padua D, Mahurkar-Joshi S, Law IK, Polytarchou C, Vu JP, Pisegna JR, et al. A long noncoding RNA signature for ulcerative colitis identifies IFNG-AS1 as an enhancer of inflammation. Am J Physiol Gastrointest Liver Physiol 2016;311:G446–G457.
Wang J, Peng H, Tian J, Ma J, Tang X, Rui K, et al. Upregulation of long noncoding RNA TMEVPG1 enhances T helper type 1 cell response in patients with Sjögren syndrome. Immunol Res 2016;64:489–96.
Hu Q, Ye Y, Chan LC, Li Y, Liang K, Lin A, et al. Oncogenic lncRNA downregulates cancer cell antigen presentation and intrinsic tumor suppression. Nat Immunol 2019;20:835–51.
Ahmad I, Naqvi RA, Valverde A, Naqvi AR. LncRNA MALAT1/microRNA-30b axis regulates macrophage polarization and function. Front Immunol. 2023;14:1214810.
Valverde A, Naqvi RA, Naqvi AR. Non-coding RNA LINC01010 regulates macrophage polarization and innate immune functions by modulating NFκB signaling pathway. J Cell Physiol. 2024;239:e31225.
Irmin FF, Oger F, Gheeraert C, Dubois-Chevalier J, Lefebvre P. The RBM14/CoAA-interacting, long intergenic non-coding RNA Paral1 regulates adipogenesis and coactivates the nuclear receptor PPARγ. Sci Rep. 2017;7:14087.
Le Naour F, Hohenkirk L, Grolleau A, Misek DE, Lescure P, Geiger JD, et al. Profiling changes in gene expression during differentiation and maturation of monocyte-derived dendritic cells using both oligonucleotide microarrays and proteomics. J Biol Chem. 2001;276:17920–31.
Gosset P, Charbonnier AS, Delerive P, Fontaine J, Staels B, Pestel J, et al. Peroxisome proliferator-activated receptor γ activators affect the maturation of human monocyte-derived dendritic cells. Eur J Immunol. 2001;31:2857–65.
Nencioni A, Grünebach F, Zobywlaski A, Denzlinger C, Brugger W, Brossart P. Dendritic Cell Immunogenicity Is Regulated by Peroxisome Proliferator-Activated Receptor γ1. J Immunol 2002;169:1228–35.
Szatmari I, Gogolak P, Im JS, Dezso B, Rajnavolgyi E, Nagy L. Activation of PPARgamma specifies a dendritic cell subtype capable of enhanced induction of iNKT cell expansion. Immunity 2004;21:95–106.
Kim S, Cho S, Kim JH. CD1-mediated immune responses in mucosal tissues: molecular mechanisms underlying lipid antigen presentation system. Exp Mol Med. 2023;55:1858–71.
Zhao JY, Yao JM, Zhang XZ, Wang KL, Jiang S, et al. A New Ferroptosis-Related Long Non-Coding RNA risk model predicts the prognosis of patients with papillary thyroid cancer. World J Oncol. 2024;4:648–61.
Cao Z, Umek RM, McKnight SL. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991;5:1538–52.
Yeh WC, Cao Z, Classon M, McKnight SL. Cascade regulation of terminal adipocyte differentiation by three members of the C/EBP family of leucine zipper proteins. Genes Dev. 1995;9:168–81.
Rosen ED, Hsu CH, Wang X, Sakai S, Freeman MW, Gonzalez FJ, et al. C/EBPalpha induces adipogenesis through PPARgamma: a unified pathway. Genes Dev. 2002;16:22–6.
Welner RS, Bararia D, Amabile G, Czibere A, Benoukraf T, et al. C/EBPα is required for development of dendritic cell progenitors. Blood. 2013;121:4073–81.
Lefterova MI, Zhang Y, Steger DJ, Schupp M, Schug J, Cristancho A, et al. PPARgamma and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes Dev. 2008;22:2941–52.
Funding
This study was supported by NIH/NIDCR contract grant numbers R03DE027147, R01DE027980, R21DE026259 (ARN) and NIH/NEI contract grant number 1R01EY033622 (DS and ARN). We acknowledge Flow cytometry core, UIC for assisting in flow cytometry analyses.
Author information
Authors and Affiliations
Contributions
RAN (Investigation, Methodology, Validation, Formal analysis, Resources, Original draft preparation, Reviewing and Editing); AV (Investigation, Methodology, Validation, Formal analysis, Resources); DS (Methodology, Resources, Writing- Original draft preparation, Reviewing and Editing); AN (Conceptualization, Investigation, Resources, Writing- Original draft preparation, Reviewing and Editing).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Naqvi, R.A., Valverde, A., Shukla, D. et al. Long noncoding RNA PARAL1 regulates myeloid dendritic cell differentiation and TLR signaling. Genes Immun 26, 151–165 (2025). https://doi.org/10.1038/s41435-025-00323-9
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41435-025-00323-9