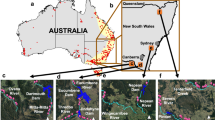
Abstract
The platypus is an evolutionary unique mammal on the east coast of mainland Australia and throughout Tasmania. The species is dependent on freshwater ecosystems, is declining throughout its range, and is listed as Vulnerable in the state of Victoria, and Near Threatened on the IUCN Red List. This relatively long-lived species is cryptic and nocturnal making it difficult to study in natural populations. Relatively little is known about its demographic history or the forces that shape genetic variation. We use a unique genomic dataset comprising 2715 single-nucleotide polymorphisms from 545 individual platypuses sampled from five catchments across Melbourne, Victoria. This dataset enabled us to describe the genetic variation across the catchments and test hypotheses relating to migration, effective population size, and potential negative effects of anthropogenic barriers. We found relatively consistent levels of genetic diversity in platypuses across Melbourne’s catchments, moderate levels of within-catchment migration, and genetic differentiation both between and within catchments. This genetic structure is explained by several factors, including isolation-by-river-distance, isolation-by-environment and within-catchment sex biased dispersal at short distances. These patterns are likely explained by a temporal lag between indirect and direct anthropogenic changes to the environmental and genetic variation, and these contemporary analyses likely reflect historical demographic patterns. In addition, we find that anthropogenic barriers such as dams have not measurably affected migration in these catchments. Our study highlights future evolutionary challenges that exist for platypuses in Melbourne’s catchments, which could be representative of their entire range along the east coast of Australia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Data used in this study are available on figshare: https://doi.org/10.6084/m9.figshare.29097086.
References
Adamack AT, Gruber B (2014) PopGenReport: simplifying basic population genetic analyses in R. Methods Ecol Evol 5:384–387
Ahrens CW, James EA (2016) Conserving the small milkwort, Comesperma polygaloides, a vulnerable subshrub in a fragmented landscape. Conserv Genet 17:891–901
Ahrens CW, Rymer PD, Stow A, Bragg J, Dillon S, Umbers KDL et al. (2018) The search for loci under selection: trends, biases and progress. Mol Ecol 27:1342–1356
Bino G, Kingsford RT, Wintle BA (2020) A stitch in time—synergistic impacts to platypus metapopulation extinction risk. Biol Conserv 242:108399
Bino G, Kingsford RT, Archer M, Connolly JH, Day J, Dias K et al. (2019) The platypus: evolutionary history, biology, and an uncertain future. J Mammal 100:308–327
Blomqvist D, Pauliny A, Larsson M, Flodin L-Å (2010) Trapped in the extinction vortex? Strong genetic effects in a declining vertebrate population. BMC Evol Biol 10:33
Bradburd GS, Coop GM, Ralph PL (2018) Inferring Continuous and Discrete Population Genetic Structure Across Space. Genetics 210:33–52
Bragg JG, Supple MA, Andrew RL, Borevitz JO (2015) Genomic variation across landscapes: insights and applications. New Phytol 207:953–967
Brunt T, Cecil M, Griffiths J, Adams-Hosking C, Murray P (2021) Where are the platypuses (Ornithorhynchus anatinus) now? A snapshot in time of their distribution in the Greater Brisbane region. Aust Mammal 43:368
Bush A, Mokany K, Catullo R, Hoffmann A, Kellermann V et al. (2016) Incorporating evolutionary adaptation in species distribution modelling reduces projected vulnerability to climate change. Ecol Lett 19:1468–1478
Butchart SH, Walpole M, Collen B, Van Strien A, Scharlemann JP, Almond RE et al. (2010) Global biodiversity: indicators of recent declines. Science 328:1164–1168
Catchen J, Hohenlohe PA, Bassham S, Amores A, Cresko WA (2013) Stacks: an analysis tool set for population genomics. Mol Ecol 22:3124–3140
Caye K, Jumentier B, Lepeule J, François O (2019) LFMM 2: fast and accurate inference of gene-environment associations in genome-wide studies. Mol Biol Evol 36:msz008
Coleman RA, Gauffre B, Pavlova A, Beheregaray LB, Kearns J, Lyon J et al. (2018) Artificial barriers prevent genetic recovery of small isolated populations of a low-mobility freshwater fish. Hered 120:515–532
Coleman RA, Chee YE, Bond NR, Weeks A, Griffiths J, Serena M et al. (2022) Understanding and managing the interactive impacts of growth in urban land use and climate change on freshwater biota: a case study using the platypus (Ornithorhynchus anatinus). Glob Chang Biol 28:1287–1300
Danecek P, Bonfield JK, Liddle J, Marshall J, Ohan V, Pollard MO et al. (2021) Twelve years of SAMtools and BCFtools. GigaScience 10: giab008
Do C, Waples RS, Peel D, Macbeth GM, Tillett BJ, Ovenden JR (2014) NeEstimator v2: re-implementation of software for the estimation of contemporary effective population size (Ne) from genetic data. Mol Ecol Resour 14:209–214
Duenas MA, Ruffhead HJ, Wakefield NH, Roberts PD, Hemming DJ, Diaz-Soltero H (2018) The role played by invasive species in interactions with endangered and threatened species in the United States: a systematic review. Biodivers Conserv 27:3171–3183
Ellis N, Smith SJ, Pitcher CR (2012) Gradient forests: calculating importance gradients on physical predictors. Ecol 93:156–168
Epps CW, Keyghobadi N (2015) Landscape genetics in a changing world: disentangling historical and contemporary influences and inferring change. Mol Ecol 24:6021–6040
Fitzpatrick MC, Keller SR (2015) Ecological genomics meets community-level modelling of biodiversity: mapping the genomic landscape of current and future environmental adaptation. Ecol Lett 18:1–16
Frankham R (2005) Genetics and extinction. Biol Conserv 126:131–140
Frankham R (2022) Evaluation of proposed genetic goals and targets for the Convention on Biological Diversity. Conserv Genet 23:865–870
Frichot E, François O (2015) LEA: an R package for landscape and ecological association studies. Methods Ecol Evol 6:925–929
Furlan E, Griffiths J, Gust N, Armistead R, Mitrovski P, Handasyde KA et al. (2012) Is body size variation in the platypus (Ornithorhynchus anatinus) associated with environmental variables?. Aust J Zool 59:201–215
Furlan EM, Griffiths J, Gust N, Handasyde KA, Grant TR, Gruber B et al. (2013) Dispersal patterns and population structuring among platypuses, Ornithorhynchus anatinus, throughout south-eastern Australia. Conserv Genet 14:837–853
Goudet J (2005) Hierfstat, a package for R to compute and test hierarchical F-statistics. Mol Ecol Notes 5:184–186
Grant TR, Temple-Smith PD (2003) Conservation of the Platypus, Ornithorhynchus anatinus: threats and challenges. Aquat Ecosyst Health Manag 6:5–18
Grant TR (1992) Historical and current distribution of the platypus, Ornithorhynchus anatinus, in Australia. In: Augee ML (ed) Platypus and echidnas. Royal Zoological Society of New South Wales, pp 232–254 (Sydney, NSW, Australia)
Gruber B, Unmack PJ, Berry OF, Georges A (2018) dartr: an r package to facilitate analysis of SNP data generated from reduced representation genome sequencing. Mol Ecol Res 18:691–699
Gust N, Griffiths J (2009) Platypus mucormycosis and its conservation implications. Aust Mycol 28:1–8.
Hawke T, Bino G, Kingsford RT (2021) Damming insights: variable impacts and implications of river regulation on platypus populations. Aquat Conserv 31:504–519
Hijmans R (2024) raster: geographic data analysis and modeling. R package version 3.6-30. https://rspatial.org/raster
Hoffman JR, Willoughby JR, Swanson BJ, Pangle KL, Zanatta DT (2017) Detection of barriers to dispersal is masked by long lifespans and large population sizes. Ecol Evol 7:9613–9623
Hoffmann AA, Sgrò CM (2011) Climate change and evolutionary adaptation. Nature 470:479–485
Hoffmann AA, Sgrò CM, Kristensen TN (2017) Revisiting adaptive potential, population size, and conservation. Trends Ecol Evol 32:506–517
Honnay O, Jacquemyn H (2007) Susceptibility of common and rare plant species to the genetic consequences of habitat fragmentation. Conserv Biol 21:823–831
James EA, Jordan R (2014) Limited structure and widespread diversity suggest potential buffers to genetic erosion in a threatened grassland shrub Pimelea spinescens (Thymelaeaceae). Conserv Genet 15:305–317
Kamvar ZN, Brooks JC, Grünwald NJ (2015) Novel R tools for analysis of genome-wide population genetic data with emphasis on clonality. Front Genet 6:208
Kardos M, Armstrong EE, Fitzpatrick SW, Hauser S, Hedrick PW, Miller JM et al. (2021) The crucial role of genome-wide genetic variation in conservation. Proc Natl Acad Sci USA 118: e2104642118
Karger DN, Conrad O, Böhner J, Kawohl T, Kreft H, Soria-Auza RW et al. (2017) Climatologies at high resolution for the Earth’s land surface areas. Sci Data 4: 170122
Keller LF, Waller DM (2002) Inbreeding effects in wild populations. Trends Ecol Evol 17:230–241
Keller LF, Biebach I, Ewing SR, Hoeck PEA (2011) The genetics of reintroductions: inbreeding and genetic drift. In: Reintroduction biology: integrating science and management (ed Ewen JG, Armstrong DP, Parker KA, Seddon PJ). Blackwell, London, pp 360–394
Kilian A, Wenzl P, Huttner E, Carling J, Xia L, Blois H et al. (2012) Data production and analysis in population genomics, methods and protocols. Methods Mol Biol 888:67–89
Landguth EL, Cushman SA, Schwartz MK, Mckelvey KS, Murphy M et al. (2010) Quantifying the lag time to detect barriers in landscape genetics. Mol Ecol 19:4179–4191
Lasky JR, Josephs EB, Morris GP (2022) Genotype–environment associations to reveal the molecular basis of environmental adaptation. Plant Cell 35:125–138
Legendre P, Fortin M, Borcard D (2015) Should the Mantel test be used in spatial analysis?. Methods Ecol Evol 6:1239–1247
Li H, Durbin R (2009) Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25:1754–1760
Li H (2013) Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv https://doi.org/10.48550/arXiv.1303.3997
Lowe WH, Allendorf FW (2010) What can genetics tell us about population connectivity?. Mol Ecol 19:3038–3051
Manel S, Schwartz MK, Luikart G, Taberlet P (2003) Landscape genetics: combining landscape ecology and population genetics. Trends Ecol Evol 18:189–197
Manthey JD, Moyle RG (2015) Isolation by environment in White-breasted Nuthatches (Sitta carolinensis) of the Madrean Archipelago sky islands: a landscape genomics approach. Mol Ecol 24:3628–3638
Marsack K, Swanson BJ (2009) A genetic analysis of the impact of generation time and road-based habitat fragmentation on Eastern Box Turtles (Terrapene c. carolina). Copeia 2009:647–652
Martin EH, Walsh CJ, Serena M, Webb JA (2014) Urban stormwater runoff limits distribution of platypus. Austral Ecol 39:337–345
Mateo-Sánchez MC, Balkenhol N, Cushman S, Pérez T, Domínguez A. et al. (2015) A comparative framework to infer landscape effects on population genetic structure: are habitat suitability models effective in explaining gene flow? Landsc Ecol 30:1405–1420
Mijangos JL, Bino G, Hawke T, Kolomyjec SH, Kingsford RT et al. (2022) Fragmentation by major dams and implications for the future viability of platypus populations. Commun Biol 5:1127
Mussmann SM, Douglas MR, Chafin TK, Douglas ME (2019) BA3-SNPs: contemporary migration reconfigured in BayesAss for next-generation sequence data. Methods Ecol Evol 10:1808–1813
Pacifici M, Santini L, Marco MD, Baisero D, Francucci L et al. (2013) Generation length for mammals. Nat Conserv 5:87–94
Reed DH, Frankham R (2003) Correlation between fitness and genetic diversity. Conserv Biol 17:230–237
Serena M, Thomas JL, Williams GA, Officer RCE (1998) Use of stream and river habitats by the platypus, Ornithorhynchus anatinus, in an urban fringe environment. Aust J Zool 46:267–282
Serena M, Williams GA, Weeks AR, Griffiths J (2014) Variation in platypus (Ornithorhynchus anatinus) life-history attributes and population trajectories in urban streams. Aust J Zool 62:223–234
Sexton JP, Hangartner SB, Hoffmann AA (2014) Genetic isolation by environment or distance: which pattern of gene flow is most common? Evol 68:1–15
Spies I, Hauser L, Jorde PE, Knutsen H, Punt AE et al. (2018) Inferring genetic connectivity in real populations, exemplified by coastal and oceanic Atlantic cod. Proc Natl Acad Sci USA 115:4945–4950
Stobo-Wilson AM, Murphy BP, Crawford HM, Dawson SJ, Dickman CR (2021) Sharing meals: predation on Australian mammals by the introduced European red fox compounds and complements predation by feral cats. Biol Conserv 261: 109284
Tyers M, Tyers M (2017) Package ‘riverdist’. R package version 0.15
Waits LP, Storfer A (2015) Basics of population genetics: quantifying neutral and adaptive genetic variation for landscape genetic studies. In: Landscape genetics (ed Balkenhol N, Cushman SA, Storfer AT, Waits LP). Wiley, New York, pp 35–57
Wang IJ, Bradburd GS (2014) Isolation by environment. Mol Ecol 23:5649–5662
Wang IJ, Glor RE, Losos JB (2013) Quantifying the roles of ecology and geography in spatial genetic divergence. Ecol Lett 16:175–182
Weber JN, Bradburd GS, Stuart YE, Stutz WE, Bolnick DI (2017) Partitioning the effects of isolation by distance, environment, and physical barriers on genomic divergence between parapatric threespine stickleback. Evolution 71:342–356
Weir BS, Cockerham CC (1984) Estimating F-Statistics for the Analysis of Population Structure. Evol 38(6) 1358–1370
Whitlock MC, McCauley DE (1999) Indirect measures of gene flow and migration: FST≠1/(4Nm+1). Heredity 82:117–125
Willi Y, Kristensen TN, Sgrò CM, Weeks AR, Ørsted M et al. (2022) Conservation genetics as a management tool: the five best-supported paradigms to assist the management of threatened species. Proc Natl Acad Sci USA 119:e2105076119
Woinarski JCZ, Burbidge AA, Harrison PL (2014) The Action Plan for Australian Mammals 2012 (CSIRO Publishing, Melbourne)
Wozney KM, Haxton TJ, Kjartanson S, Wilson CC (2011) Genetic assessment of lake sturgeon (Acipenser fulvescens) population structure in the Ottawa River. Environ Biol Fishes 90:183–195
Wright PGR, Schofield H, Mathews F (2021) Can effective population size estimates be used to monitor population trends of woodland bats? A case study of Myotis bechsteinii. Ecol Evol 11:2015–2023
Wright S (1943) Isolation by distance. Genetics 28:114–138
Acknowledgements
We thank the three anonymous reviewers and the editor for their helpful comments. We thank Melbourne Water for funding the Urban Platypus Program, which provided most of the samples analysed in this study. We also thank the many volunteers who contributed to the collection of platypus samples through this program. Rebecca Jordan is thanked for organising and extracting some of the samples analysed in this study.
Author information
Authors and Affiliations
Contributions
CWA conceptualised the study, performed the analyses, generated the figures, and drafted the manuscript. JG and EF conducted fieldwork. AvR and EF performed lab work. AD and RC funded the project and helped with conceptualisation. ARW conceptualised the study and drafted the manuscript. All authors contributed to the writing and editorial process.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Research ethics statement
Live-trapping surveys undertaken by Cesar Australia were authorised by the following permits DEPI/ DEDJTR/DELWP Wildlife & Small Animal Institutions AEC—09.07, 17.10, 16.13, 24.16, 10.20 DSE/DELWP Wildlife Research permit—10004130, 10005488, 10006851, 10007966, 10009610 and DEPI/DEDJTR/VFA Fisheries General Research permit—RP907, RP1430.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Associate editor: Sam Banks.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ahrens, C.W., Griffiths, J., Danger, A. et al. Genetic diversity and structure lag the effects of contemporary environmental changes in a platypus meta-population. Heredity 134, 427–438 (2025). https://doi.org/10.1038/s41437-025-00774-w
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41437-025-00774-w